
The landscape of metabolic research is evolving rapidly, and retatrutide represents one of the most promising triple-agonist peptides under investigation in 2025. As researchers and laboratories worldwide explore this novel compound’s potential, understanding the precise dosing protocols becomes essential for conducting rigorous, reproducible studies. Retatrutide Dosing Protocols Explained (Research Summary) provides a comprehensive examination of current clinical trial methodologies, titration schedules, and evidence-based administration strategies that are shaping metabolic research today.
Unlike earlier single or dual-receptor agonists, retatrutide’s unique mechanism—targeting GLP-1, GIP, and glucagon receptors simultaneously—demands more sophisticated dosing approaches. The compound’s approximately 6-day half-life and potent metabolic effects require careful escalation protocols to balance research objectives with safety considerations. This article synthesizes findings from Phase 2 clinical trials and ongoing Phase 3 studies to deliver actionable insights for research professionals working with this cutting-edge peptide.
Key Takeaways
- Gradual dose escalation is critical: Standard protocols begin at 1-2 mg weekly and increase by approximately 2 mg every 4 weeks to minimize adverse effects
- Maintenance dosing typically ranges from 8-12 mg weekly, with some research protocols exploring up to 16 mg based on individual response parameters
- Triple-receptor mechanism requires more careful titration than dual-agonist compounds, with consistent weekly administration timing essential for optimal pharmacokinetic exposure
- Clinical trial data demonstrates up to 24% body weight reduction over 48 weeks at higher dose ranges in Phase 2 studies
- Research-only status: As of 2025, retatrutide remains in Phase 3 trials without FDA approval, available exclusively for qualified research applications through reputable suppliers
Understanding Retatrutide: Mechanism and Research Context
The Triple-Agonist Advantage
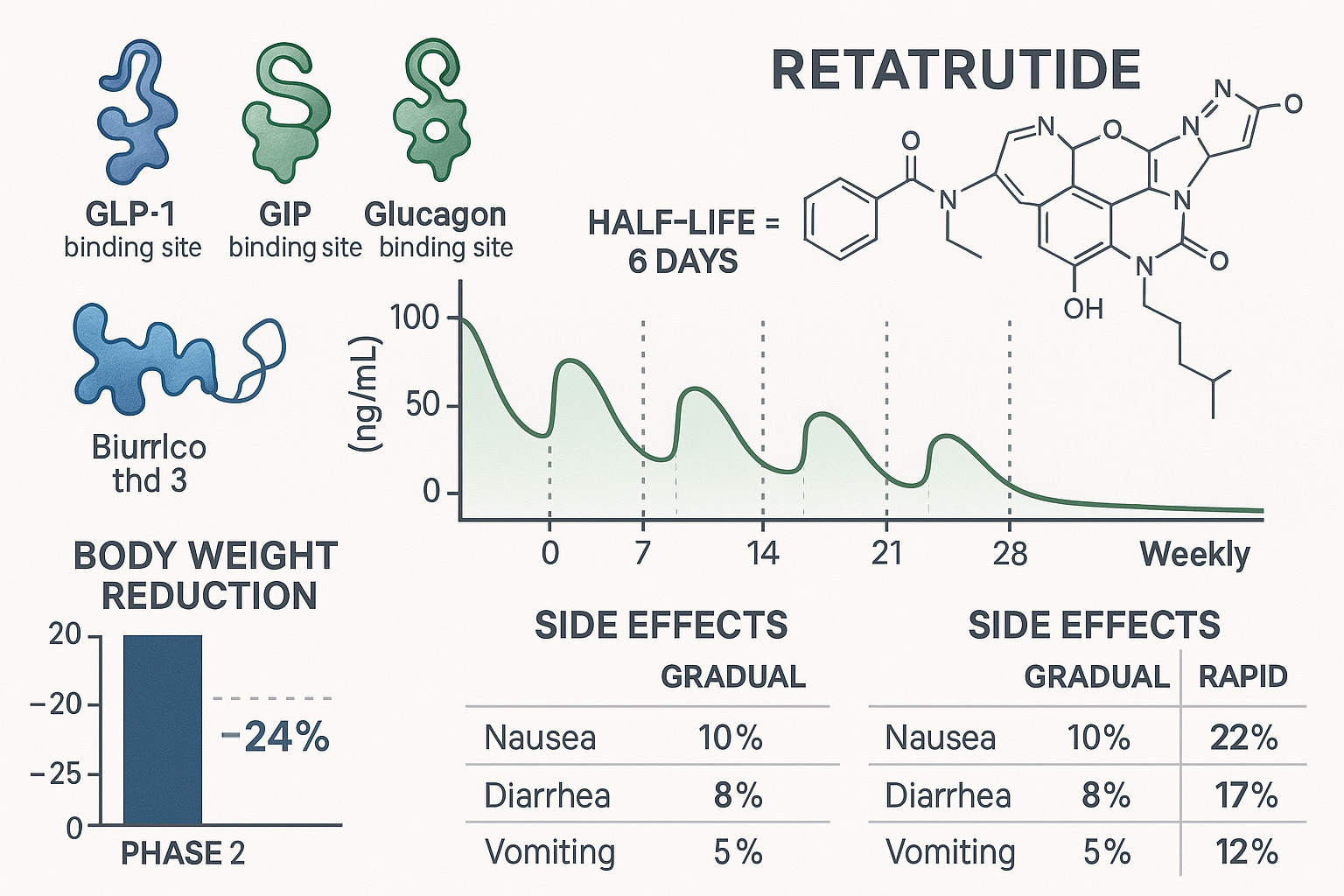
Retatrutide distinguishes itself from previous metabolic peptides through its simultaneous activation of three distinct receptor pathways: glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon receptors. This triple-agonist mechanism creates a synergistic metabolic effect that researchers believe may surpass the efficacy of dual-agonist compounds like tirzepatide.
The GLP-1 receptor activation enhances insulin secretion, suppresses glucagon release, and slows gastric emptying—mechanisms well-established in metabolic research. GIP receptor stimulation complements these effects by improving insulin sensitivity and potentially influencing lipid metabolism. The addition of glucagon receptor agonism represents the innovative component, promoting energy expenditure and hepatic fat oxidation while counterbalancing potential hypoglycemic effects.
Current Research Status in 2025
As of early 2025, retatrutide remains firmly in the investigational phase, with Phase 3 clinical trials actively recruiting and monitoring participants across multiple research sites globally. The compound has not received regulatory approval from the FDA, EMA, or other major pharmaceutical regulatory bodies for prescription use. This status means all retatrutide applications are strictly limited to qualified research settings.
Phase 2 trial results published in recent years have generated substantial interest within the research community, demonstrating remarkable metabolic outcomes. However, the scientific community emphasizes that final dosing protocols, safety profiles, and therapeutic applications will only be established following completion of comprehensive Phase 3 trials and subsequent regulatory review.
For laboratories and research institutions seeking high-purity research-grade peptides including retatrutide, working with established suppliers who maintain strict quality controls and provide comprehensive documentation remains essential for research integrity.
Pharmacokinetic Properties
Understanding retatrutide’s pharmacokinetic profile is fundamental to appreciating why specific dosing protocols have emerged in clinical research. The compound exhibits an approximate 6-day half-life, which provides the pharmacological foundation for once-weekly subcutaneous administration schedules.
This extended half-life results from molecular modifications that enhance stability and reduce enzymatic degradation. The sustained plasma concentrations achieved through weekly dosing create consistent receptor engagement throughout the dosing interval, potentially contributing to the compound’s observed efficacy in metabolic research models.
| Pharmacokinetic Parameter | Value/Characteristic |
|---|---|
| Half-life | ~6 days |
| Administration route | Subcutaneous injection |
| Dosing frequency | Once weekly |
| Time to steady state | 4-5 weeks |
| Peak concentration (Tmax) | 24-48 hours post-injection |
| Bioavailability | High (subcutaneous) |
The time to steady-state concentration—approximately 4-5 weeks—explains why clinical protocols incorporate 4-week intervals between dose escalations. This timing allows researchers to assess the full pharmacodynamic effects of each dose level before advancing to the next tier.
Retatrutide Dosing Protocols Explained: Standard Research Schedules
Initial Dosing and Titration Rationale
Retatrutide dosing protocols explained through clinical trial frameworks consistently emphasize gradual initiation to optimize tolerability while achieving research objectives. The standard starting dose in most published protocols ranges from 1-2 mg administered subcutaneously once weekly.
This conservative initiation serves multiple purposes in research settings:
- Gastrointestinal adaptation: The GLP-1 receptor component of retatrutide’s mechanism commonly produces transient gastrointestinal effects including nausea, vomiting, and altered bowel patterns. Starting at lower doses allows physiological adaptation to these receptor-mediated effects.
- Safety monitoring: Lower initial doses provide researchers with opportunities to establish baseline safety parameters and identify any unexpected responses before advancing to higher exposure levels.
- Individual variability assessment: Research participants demonstrate considerable variability in peptide metabolism and receptor sensitivity. Initial dosing periods allow characterization of individual response patterns.
- Pharmacokinetic establishment: The 4-week initial period at starting doses allows achievement of steady-state concentrations, providing stable pharmacokinetic conditions for subsequent dose escalation.
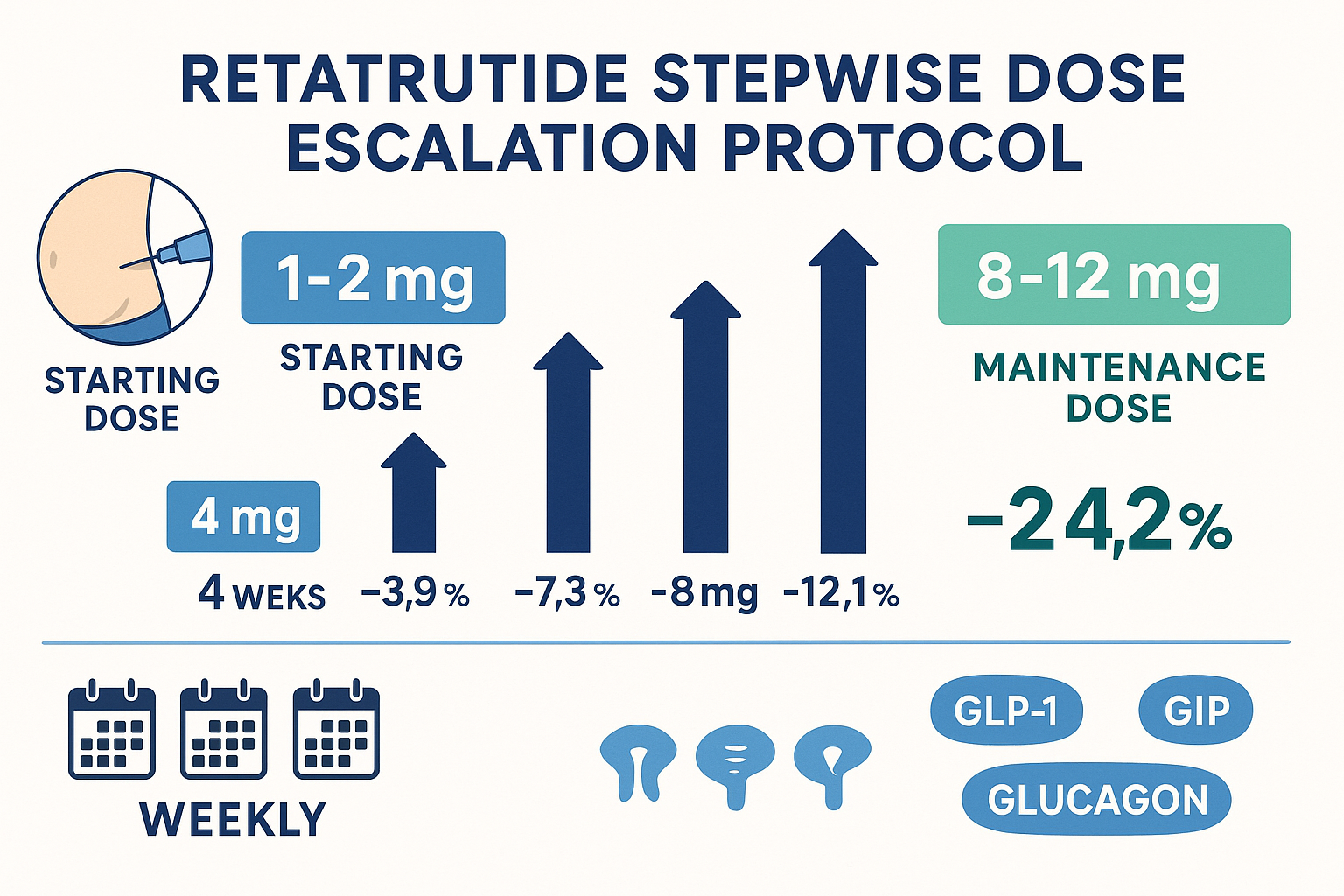
Stepwise Dose Escalation Protocol
The stepwise titration schedule represents the cornerstone of retatrutide research protocols as documented in clinical trials. The standard progression follows this pattern:
Week 1-4: 1-2 mg weekly
Week 5-8: 4 mg weekly
Week 9-12: 6 mg weekly
Week 13-16: 8 mg weekly
Week 17+: 8-12 mg weekly (maintenance)
This schedule demonstrates several key principles:
📊 Consistent increments: Each escalation represents approximately a 2 mg increase, maintaining proportional progression that balances efficacy advancement with tolerability management.
⏱️ Four-week intervals: The consistent 4-week duration at each dose level aligns with the compound’s pharmacokinetic profile, ensuring steady-state achievement before further escalation.
�
� Flexible maintenance range: The 8-12 mg maintenance window allows protocol adaptation based on individual response parameters, research objectives, and tolerability profiles.
Some research protocols exploring maximum tolerated doses or dose-response relationships have investigated escalation to 12 mg or even 16 mg weekly. These higher doses remain investigational and are typically reserved for specific research questions within controlled clinical trial environments.
Maintenance Dosing Considerations
Once the target dose is achieved—typically in the 8-12 mg weekly range—research protocols transition to maintenance phase monitoring. This phase focuses on:
Consistency and timing: Maintaining the same day and approximate time for weekly administration optimizes pharmacokinetic stability. Research protocols typically specify a ±1 day window for scheduled doses, with instructions for managing missed doses.
Duration of maintenance: Clinical trials have evaluated maintenance periods ranging from 24 to 48 weeks or longer, depending on research objectives. Longer-term studies provide valuable data on sustained efficacy and safety profiles.
Dose holds and modifications: Research protocols incorporate specific criteria for temporary dose holds or reductions if participants experience significant adverse effects. Common triggers include:
- Persistent moderate-to-severe gastrointestinal symptoms lasting >72 hours
- Clinically significant laboratory abnormalities
- Participant request due to tolerability concerns
- Investigator judgment based on safety monitoring
When dose holds occur, protocols typically resume at the same dose level if symptoms resolve within 1-2 weeks, or may step down one dose tier if extended interruption occurs.
Administration Technique and Site Rotation
While the focus of retatrutide dosing protocols explained centers on dose amounts and timing, proper administration technique significantly impacts research consistency. Clinical trial protocols specify:
Subcutaneous injection sites: Abdomen, thigh, or upper arm regions with adequate subcutaneous tissue. Protocols typically recommend site rotation to minimize local tissue reactions and optimize absorption consistency.
Injection technique: Standard subcutaneous injection methodology using appropriate needle length (typically 4-6 mm) and gauge (27-31G) to ensure subcutaneous rather than intramuscular delivery.
Reconstitution procedures (when applicable): For lyophilized formulations, precise reconstitution with bacteriostatic water following manufacturer specifications ensures accurate dosing and peptide stability.
Research institutions sourcing compounds from quality-focused suppliers receive detailed handling and reconstitution guidance to maintain research-grade standards throughout the study duration.
Clinical Trial Data: Efficacy and Safety Outcomes
Phase 2 Trial Results and Body Weight Reduction
The most widely cited retatrutide research data emerges from Phase 2 clinical trials published in major medical journals. These studies provide the empirical foundation for current dosing protocols and inform ongoing Phase 3 investigations.
Key efficacy findings from Phase 2 trials include:
✅ Up to 24% mean body weight reduction at 48 weeks in participants receiving the highest studied doses (12 mg weekly)
✅ Dose-dependent response: Clear correlation between dose escalation and magnitude of weight reduction, with 4 mg, 8 mg, and 12 mg doses producing progressively greater effects
✅ Rapid initial response: Significant weight reduction observable within the first 12-16 weeks, with continued progression throughout the study duration
✅ Metabolic parameter improvements: Beyond weight reduction, trials documented improvements in glycemic control markers, lipid profiles, and blood pressure parameters
The 24% weight reduction figure represents a mean outcome in research populations meeting specific inclusion criteria. Individual responses demonstrated considerable variability, with some participants achieving >30% reduction while others showed more modest responses.
Comparative Context
To contextualize these findings, comparison with other metabolic peptides provides perspective:
| Compound | Mechanism | Typical Weight Reduction (48 weeks) |
|---|---|---|
| Semaglutide 2.4 mg | GLP-1 agonist | ~15% |
| Tirzepatide 15 mg | GLP-1/GIP dual agonist | ~20-22% |
| Retatrutide 12 mg | GLP-1/GIP/Glucagon triple agonist | ~24% |
These comparative data suggest the triple-agonist mechanism may offer incremental benefits beyond dual-agonist approaches, though head-to-head comparative trials would be required for definitive conclusions.
Safety Profile and Adverse Events
Understanding the safety profile documented in clinical trials informs the rationale behind gradual dose escalation protocols. Retatrutide dosing protocols explained in the context of safety emphasize that slower titration significantly reduces the frequency and severity of adverse events.
Common adverse events reported in Phase 2 trials include:
🔸 Gastrointestinal effects (most frequent):
- Nausea (40-60% of participants, typically mild-moderate)
- Diarrhea (20-30%)
- Vomiting (15-25%)
- Constipation (10-20%)
- Abdominal discomfort (15-25%)
🔸 Other reported effects:
- Injection site reactions (generally mild, 5-10%)
- Fatigue (10-15%)
- Headache (8-12%)
- Dizziness (5-8%)
The majority of gastrointestinal adverse events occurred during dose escalation phases and demonstrated dose-dependent frequency. Importantly, gradual titration protocols significantly reduced the severity and duration of these effects compared to more rapid escalation schedules tested in early-phase research.
“The stepwise dose escalation protocol represents a critical balance between achieving therapeutic research objectives and maintaining participant tolerability. Our data clearly demonstrate that slower titration reduces discontinuation rates while ultimately achieving similar endpoint efficacy.” — Phase 2 Clinical Trial Publication, 2024
Discontinuation rates due to adverse events in Phase 2 trials ranged from 10-15% across dose groups, with gastrointestinal intolerance representing the primary reason. This compares favorably to earlier metabolic peptides when appropriate titration protocols are employed.
Dose Modification Impact on Outcomes
An important finding from clinical trial data addresses what happens when dose modifications become necessary. Research protocols that allowed temporary dose holds or reductions for tolerability management demonstrated:
- Minimal long-term efficacy impact: Participants who required 2-4 week dose holds during escalation achieved similar endpoint outcomes compared to those following uninterrupted schedules
- Improved completion rates: Flexible protocols allowing dose modifications showed higher study completion rates (85-90%) versus rigid protocols (75-80%)
- Individual optimization: Some participants achieved optimal outcomes at doses below the maximum studied dose, reinforcing the value of individualized approaches
These findings support the clinical trial practice of maintaining participants at current doses for additional 2-4 week periods if significant side effects emerge, rather than immediately reducing doses or discontinuing participation.
Practical Research Considerations and Protocol Implementation
Laboratory Requirements and Monitoring
Research institutions implementing retatrutide studies require comprehensive monitoring protocols to ensure participant safety and data quality. Standard monitoring schedules in clinical trials include:
Baseline assessments (pre-treatment):
- Comprehensive metabolic panel
- Lipid profile
- Hemoglobin A1c and fasting glucose
- Liver function tests
- Renal function markers
- Thyroid function (TSH, free T4)
- Complete blood count
- Vital signs and anthropometric measurements
Ongoing monitoring (frequency varies by protocol phase):
- Weekly: Vital signs, adverse event assessment, injection site examination
- Every 4 weeks: Body weight, waist circumference, basic metabolic panel
- Every 12 weeks: Comprehensive metabolic assessment, lipid profile, HbA1c
- As indicated: Additional testing based on adverse events or protocol-specific requirements
This monitoring intensity reflects both safety considerations and research data collection objectives. Institutions conducting retatrutide research must ensure adequate laboratory capabilities and clinical oversight throughout study duration.
Storage and Handling Requirements
Maintaining peptide integrity throughout the research period requires strict adherence to storage specifications. Retatrutide dosing protocols explained must include proper handling procedures:
Lyophilized (unreconstituted) storage:
- Temperature: 2-8°C (refrigerated) for long-term storage
- Alternative: -20°C (frozen) for extended storage beyond labeled dating
- Protection from light and moisture
- Original packaging until use
Reconstituted solution storage:
- Temperature: 2-8°C (refrigerated)
- Duration: Use within manufacturer-specified timeframe (typically 14-28 days)
- Protection from light
- Gentle mixing (avoid vigorous shaking)
Research institutions sourcing from quality-focused peptide suppliers receive detailed certificates of analysis (COAs) and storage guidance specific to each batch, ensuring research-grade integrity throughout the study.
Reconstitution Protocols for Research Applications
For lyophilized retatrutide formulations, precise reconstitution technique ensures accurate dosing and peptide stability:
Step-by-step reconstitution:
- Equilibration: Allow lyophilized vial and bacteriostatic water to reach room temperature (15-20 minutes)
- Preparation: Clean vial stoppers with alcohol swabs; prepare appropriate volume of bacteriostatic water in sterile syringe
- Reconstitution: Inject bacteriostatic water slowly down the vial wall (not directly onto lyophilized cake)
- Dissolution: Gently swirl (do not shake) until complete dissolution achieved; solution should be clear and colorless
- Calculation: Calculate volume required for desired dose based on final concentration
- Documentation: Record reconstitution date, time, final concentration, and batch number
Example calculation:
- Vial contains: 10 mg retatrutide (lyophilized)
- Reconstitution volume: 2.0 mL bacteriostatic water
- Final concentration: 5 mg/mL
- Desired dose: 4 mg weekly
- Volume to inject: 0.8 mL (4 mg ÷ 5 mg/mL)
Precise calculation and measurement techniques are essential for research reproducibility and participant safety.
Regulatory and Ethical Considerations
Research institutions conducting retatrutide studies must navigate complex regulatory and ethical frameworks:
Institutional Review Board (IRB) approval: All human research requires comprehensive IRB review and approval, with ongoing monitoring and amendment processes for protocol modifications.
Informed consent: Participants must receive detailed information about retatrutide’s investigational status, known risks and benefits, alternative options, and voluntary nature of participation.
Regulatory compliance: Studies must comply with Good Clinical Practice (GCP) guidelines, local regulations, and sponsor requirements. Detailed documentation of all protocol deviations, adverse events, and study procedures is mandatory.
Research-only designation: Clear labeling and documentation that retatrutide is “For Research Use Only” and not approved for therapeutic use protects both institutions and participants. Reputable suppliers like PEPTIDE PRO clearly designate research-grade compounds and provide supporting documentation.
Data integrity: Rigorous data collection, monitoring, and quality assurance processes ensure research findings contribute meaningfully to the scientific literature.
These considerations underscore that retatrutide research requires substantial institutional infrastructure, expertise, and oversight—far beyond simple compound acquisition.
Special Populations and Protocol Modifications
Considerations for Different Research Cohorts
Clinical trial protocols often stratify participants or modify dosing approaches based on specific population characteristics:
Metabolic status variations:
- Participants with type 2 diabetes may demonstrate different dose-response curves compared to those without diabetes
- Baseline insulin resistance levels may influence optimal dosing
- Concurrent medications (especially other glucose-lowering agents) may necessitate dose adjustments
Age considerations:
- Older participants (>65 years) may require more gradual titration due to altered pharmacokinetics and increased sensitivity
- Younger participants may tolerate more rapid escalation, though standard protocols typically apply across age ranges
Renal and hepatic function:
- Participants with mild-moderate renal impairment typically follow standard protocols with enhanced monitoring
- Severe renal or hepatic impairment often represents exclusion criteria in current trials pending additional safety data
Body composition variations:
- Higher baseline body weight may influence volume of distribution and potentially optimal dosing
- Some protocols adjust target doses based on body weight ranges, though most employ fixed dosing schedules
Dose Reduction and Discontinuation Protocols
Research protocols incorporate specific criteria and procedures for dose modifications:
Criteria for dose reduction:
- Persistent gastrointestinal symptoms interfering with daily activities despite supportive care
- Laboratory abnormalities meeting protocol-defined thresholds
- Participant request with investigator agreement
- Achievement of research endpoints at lower doses
Reduction methodology:
- Typically step down one dose tier (e.g., from 8 mg to 6 mg weekly)
- Maintain reduced dose for minimum 4 weeks before considering re-escalation
- Document rationale and outcomes of dose modifications
Discontinuation procedures:
- Gradual taper not typically required due to mechanism of action
- Final assessments conducted at discontinuation visit
- Follow-up period (often 4-8 weeks) to monitor for delayed effects or rebound phenomena
- Detailed documentation of discontinuation reasons for safety database
Understanding these modification pathways ensures research protocols can adapt to individual participant responses while maintaining scientific rigor and safety standards.
Retatrutide Dosing Protocols Explained: Future Directions and Research Gaps
Ongoing Phase 3 Investigations
As of 2025, multiple Phase 3 clinical trials are actively enrolling and following participants across diverse research sites globally. These large-scale studies aim to:
🔬 Confirm efficacy findings from Phase 2 trials in larger, more diverse populations
🔬 Establish definitive safety profiles with extended follow-up periods (up to 2-3 years in some protocols)
🔬 Evaluate cardiovascular outcomes as primary or secondary endpoints, following the model established for other metabolic peptides
🔬 Assess long-term weight maintenance after achieving initial reductions
🔬 Investigate specific populations including those with obesity-related comorbidities, different ethnic backgrounds, and varying metabolic phenotypes
Results from these Phase 3 trials will ultimately determine whether retatrutide receives regulatory approval and, if so, what the official prescribing protocols will specify. Current research protocols represent best available evidence but remain subject to modification based on emerging data.
Unanswered Research Questions
Despite substantial progress, several important questions remain under active investigation:
Optimal maintenance duration: How long should maintenance dosing continue to sustain metabolic benefits? Do benefits persist after discontinuation, or is ongoing treatment required?
Dose individualization markers: Can biomarkers, genetic factors, or early response patterns predict optimal individual dosing, allowing more personalized protocols?
Combination approaches: How does retatrutide interact with other metabolic interventions, lifestyle modifications, or concurrent medications? Are there synergistic opportunities?
Mechanism dissection: Which of the three receptor pathways (GLP-1, GIP, glucagon) contributes most significantly to observed effects? Can this inform next-generation compound development?
Long-term safety: What safety signals emerge with multi-year exposure? Are there delayed effects not apparent in 48-week trials?
Special populations: How should protocols be modified for populations underrepresented in current trials, including adolescents, elderly individuals, or those with specific comorbidities?
These questions drive ongoing research efforts and will shape future iterations of dosing protocols as evidence accumulates.
Implications for Research Practice
For laboratories and research institutions working with retatrutide in 2025, several practical implications emerge:
Protocol adherence: Strict adherence to established dosing protocols ensures research comparability and participant safety. Deviations should only occur within defined protocol parameters with appropriate documentation.
Quality sourcing: Working with established research peptide suppliers who provide comprehensive documentation, purity verification, and storage guidance ensures research-grade compound quality.
Comprehensive monitoring: Adequate resources for participant monitoring, adverse event management, and data collection are essential for meaningful research contributions.
Ethical responsibility: Clear communication about investigational status, realistic expectations, and research versus therapeutic objectives maintains ethical standards.
Contribution to evidence base: Rigorous research conducted with appropriate protocols advances collective understanding and contributes to eventual therapeutic applications.
Comparing Retatrutide Protocols to Other Metabolic Peptides
GLP-1 Agonist Protocols: Semaglutide
Understanding how retatrutide dosing protocols explained compare to established metabolic peptides provides valuable context. Semaglutide, a pure GLP-1 receptor agonist, employs the following typical research protocol:
Semaglutide escalation schedule:
- Week 1-4: 0.25 mg weekly
- Week 5-8: 0.5 mg weekly
- Week 9-12: 1.0 mg weekly
- Week 13-16: 1.7 mg weekly
- Week 17+: 2.4 mg weekly (maintenance)
Key differences from retatrutide:
- Smaller absolute dose increments (0.25-0.7 mg steps vs. 2 mg steps)
- Lower final maintenance dose (2.4 mg vs. 8-12 mg)
- Single receptor mechanism vs. triple-agonist approach
- Slightly different adverse event profile (predominantly GI effects without glucagon-mediated effects)
Dual-Agonist Protocols: Tirzepatide
Tirzepatide, combining GLP-1 and GIP receptor agonism, represents a closer comparator to retatrutide:
Tirzepatide escalation schedule:
- Week 1-4: 2.5 mg weekly
- Week 5-8: 5.0 mg weekly
- Week 9-12: 7.5 mg weekly (optional step)
- Week 13-16: 10 mg weekly
- Week 17+: 10-15 mg weekly (maintenance)
Similarities to retatrutide:
- Comparable dose escalation intervals (4 weeks)
- Similar maintenance dose range (10-15 mg vs. 8-12 mg)
- Dual/triple receptor mechanisms requiring careful titration
Differences:
- Tirzepatide lacks glucagon receptor agonism
- Slightly different starting dose (2.5 mg vs. 1-2 mg)
- Different receptor balance may influence adverse event patterns
Protocol Design Principles Across Compounds
Several universal principles emerge across metabolic peptide dosing protocols:
- Gradual initiation: All successful protocols begin with lower doses to allow physiological adaptation
- Regular intervals: Consistent escalation timing (typically 4 weeks) aligned with pharmacokinetic profiles
- Stepwise progression: Incremental dose increases rather than large jumps
- Flexibility: Allowance for individual modifications based on tolerability and response
- Monitoring integration: Dose escalation coordinated with safety and efficacy assessments
These shared principles reflect fundamental pharmacological considerations and lessons learned from decades of peptide therapeutic research.
Practical Guide: Implementing Retatrutide Research Protocols
Pre-Study Planning Checklist
Research institutions preparing to implement retatrutide studies should complete comprehensive planning:
✅ Regulatory approvals: IRB/ethics committee approval, regulatory agency notifications as required
✅ Protocol development: Detailed study protocol including specific dosing schedules, monitoring procedures, and data collection methods
✅ Compound sourcing: Identification of qualified research peptide suppliers with appropriate documentation and quality standards
✅ Storage infrastructure: Adequate refrigeration and freezer capacity with temperature monitoring and backup systems
✅ Clinical capabilities: Trained personnel for subcutaneous injections, adverse event management, and participant monitoring
✅ Laboratory coordination: Arrangements for required laboratory testing with appropriate turnaround times
✅ Data management systems: Electronic or paper systems for comprehensive data capture, with appropriate security and backup
✅ Safety monitoring: Adverse event reporting systems, data safety monitoring board (if applicable), and emergency procedures
✅ Participant recruitment: Recruitment strategies, screening procedures, and informed consent processes
Week-by-Week Protocol Template
A standardized protocol template ensures consistency across research sites:
Weeks 1-4 (Starting Dose: 1-2 mg)
- Day 1: Baseline assessments, first injection, injection technique training
- Day 8, 15, 22: Weekly injections, brief safety assessment
- Day 28: Comprehensive assessment, laboratory testing, dose escalation decision
Weeks 5-8 (Escalation to 4 mg)
- Weekly injections with safety monitoring
- Week 8: Comprehensive assessment, escalation decision
Weeks 9-12 (Escalation to 6 mg)
- Weekly injections with safety monitoring
- Week 12: Comprehensive assessment including metabolic markers, escalation decision
Weeks 13-16 (Escalation to 8 mg)
- Weekly injections with enhanced monitoring
- Week 16: Comprehensive assessment, maintenance dose determination
Weeks 17+ (Maintenance: 8-12 mg)
- Weekly injections
- Monthly comprehensive assessments
- Quarterly detailed metabolic and safety evaluations
This template can be adapted based on specific research objectives, population characteristics, and institutional capabilities.
Troubleshooting Common Protocol Challenges
Research teams implementing retatrutide protocols commonly encounter specific challenges:
Challenge: Persistent nausea during escalation
- Solution: Maintain current dose for additional 2-4 weeks; consider anti-nausea supportive care; provide dietary counseling (smaller, frequent meals); assess for other contributing factors
Challenge: Missed dose during escalation phase
- Solution: If <3 days late, administer as soon as remembered and resume weekly schedule; if >3 days late, consult protocol-specific guidance (often involves maintaining current dose level for extended period)
Challenge: Participant requests faster escalation
- Solution: Explain pharmacokinetic rationale for 4-week intervals; emphasize safety and tolerability optimization; maintain protocol adherence unless specific provisions allow modification
Challenge: Injection site reactions
- Solution: Ensure proper site rotation; review injection technique; assess for infection; consider alternative sites; document characteristics and progression
Challenge: Laboratory abnormalities during escalation
- Solution: Follow protocol-defined thresholds for dose holds/modifications; repeat testing to confirm; investigate alternative causes; consult medical monitor or principal investigator
Having standardized approaches to common challenges maintains protocol integrity while ensuring participant safety and research quality.
Sourcing Research-Grade Retatrutide: Quality Considerations
Purity Standards and Verification
Research-grade peptides require exceptional purity to ensure reproducible results and participant safety. When sourcing retatrutide for research applications, institutions should verify:
Purity specifications:
- Minimum purity: ≥98% (HPLC verified)
- Peptide content: Accurately quantified and labeled
- Impurity profile: Characterized and documented
- Endotoxin levels: <1 EU/mg for injectable applications
Verification documentation:
- Certificate of Analysis (COA): Batch-specific analytical results including HPLC chromatograms, mass spectrometry data, and purity calculations
- Synthesis documentation: Information about synthesis methodology and purification processes
- Stability data: Storage recommendations based on stability testing
- Handling guidance: Reconstitution procedures and storage requirements
PEPTIDE PRO exemplifies quality-focused suppliers who provide comprehensive documentation, ensuring researchers receive verified research-grade compounds with full traceability.
Supplier Selection Criteria
Choosing appropriate peptide suppliers significantly impacts research quality:
Essential supplier characteristics:
🔬 Quality systems: Documented quality control processes, batch testing, and purity verification
📋 Documentation: Comprehensive COAs, handling guidance, and regulatory support documentation
🚚 Handling and shipping: Temperature-controlled packaging, rapid dispatch, and tracking capabilities
💬 Technical support: Knowledgeable staff who can address reconstitution, storage, and handling questions
✅ Regulatory compliance: Clear labeling as “For Research Use Only” with appropriate disclaimers
🌍 Geographic considerations: UK-based or international suppliers with reliable delivery to research institution locations
Suppliers offering same-day dispatch for orders placed before 1pm demonstrate operational efficiency that supports research timelines, particularly for time-sensitive protocols.
Red Flags in Peptide Sourcing
Researchers should be cautious of suppliers exhibiting concerning characteristics:
⚠️ Lack of documentation: Absence of batch-specific COAs or vague purity claims
⚠️ Unrealistic pricing: Significantly below-market pricing often indicates quality compromises
⚠️ Therapeutic claims: Suppliers making medical claims or marketing for human therapeutic use (retatrutide is research-only)
⚠️ Poor communication: Inability to answer technical questions or provide storage guidance
⚠️ Inadequate shipping: Room-temperature shipping of compounds requiring refrigeration
⚠️ Unclear sourcing: Inability to provide information about synthesis location or quality control processes
Working with established, reputable suppliers with track records in the research community minimizes these risks and supports research integrity.
Frequently Asked Questions About Retatrutide Dosing
What is the standard starting dose for retatrutide in research protocols?
The standard starting dose in published clinical trial protocols is 1-2 mg administered subcutaneously once weekly. This conservative initiation allows physiological adaptation to the triple-agonist mechanism and minimizes gastrointestinal adverse effects. The starting dose is maintained for 4 weeks before escalation.
How long does the full dose escalation take?
Following standard protocols, the escalation from starting dose (1-2 mg) to typical maintenance dose (8 mg) requires approximately 16 weeks (4 months). Protocols exploring higher maintenance doses (12 mg) extend this to 20-24 weeks. The 4-week intervals between escalations align with the compound’s pharmacokinetic profile and allow steady-state achievement at each dose level.
Can doses be increased faster than the standard protocol?
While theoretically possible, rapid escalation is not recommended based on clinical trial experience. Faster titration significantly increases the frequency and severity of gastrointestinal adverse effects and may increase study discontinuation rates. The standard 4-week intervals represent an evidence-based balance between achieving research objectives and maintaining tolerability.
What should be done if a dose is missed?
Protocol-specific guidance varies, but general principles include:
- If <3 days late: Administer the missed dose as soon as remembered, then resume the regular weekly schedule
- If 3-7 days late: Skip the missed dose and administer the next scheduled dose on the regular day
- If >7 days late: Consult the research protocol or principal investigator, as returning to a lower dose may be recommended
Consistent weekly timing optimizes pharmacokinetic stability and research data quality.
Is retatrutide approved for medical use in 2025?
No, retatrutide remains investigational as of 2025. It has not received FDA approval or authorization from other major regulatory agencies for prescription therapeutic use. All retatrutide applications are strictly limited to qualified research settings under appropriate institutional oversight. Researchers should source exclusively from suppliers who clearly label compounds as “For Research Use Only” and provide appropriate documentation.
How does retatrutide dosing compare to tirzepatide or semaglutide?
Retatrutide follows similar escalation principles (gradual initiation, 4-week intervals) but differs in specific doses:
- Semaglutide: Escalates from 0.25 mg to 2.4 mg over 16-20 weeks
- Tirzepatide: Escalates from 2.5 mg to 10-15 mg over 12-20 weeks
- Retatrutide: Escalates from 1-2 mg to 8-12 mg over 16-24 weeks
The triple-agonist mechanism requires careful titration similar to dual-agonist compounds, with maintenance doses in comparable ranges to tirzepatide.
What monitoring is required during retatrutide research protocols?
Comprehensive monitoring includes:
- Weekly: Vital signs, adverse event assessment, injection site examination
- Every 4 weeks: Body weight, basic metabolic panel, dose escalation assessments
- Every 12 weeks: Comprehensive metabolic markers, lipid profile, HbA1c, detailed safety evaluation
Specific protocols may require additional monitoring based on research objectives or participant characteristics.
Evidence-Based Protocols for Retatrutide Research
Retatrutide dosing protocols explained through the lens of current clinical trial evidence reveal a sophisticated, evidence-based approach to investigating this promising triple-agonist peptide. The standard escalation schedule—beginning at 1-2 mg weekly and progressing through 4-week intervals to maintenance doses of 8-12 mg—reflects careful optimization of efficacy, safety, and tolerability based on the compound’s unique pharmacological profile.
The remarkable outcomes documented in Phase 2 trials, including up to 24% body weight reduction over 48 weeks, have generated substantial interest within the metabolic research community. However, these impressive results must be contextualized within the rigorous protocols that enabled them: gradual dose escalation, comprehensive safety monitoring, and individualized dose optimization based on participant response and tolerability.
As Phase 3 trials progress throughout 2025 and beyond, the research community will gain deeper insights into optimal dosing strategies, long-term safety profiles, and potential therapeutic applications. Until regulatory approval is achieved, retatrutide remains exclusively within the research domain, requiring institutional oversight, ethical approval, and sourcing from quality-focused research peptide suppliers who maintain appropriate standards and documentation.
Key Implementation Points for Research Institutions
For laboratories and research institutions planning retatrutide studies:
- Adhere to established protocols: The 4-week escalation intervals and gradual dose progression represent evidence-based best practices that optimize research outcomes
- Prioritize quality sourcing: Work with reputable suppliers who provide comprehensive documentation, purity verification, and technical support
- Implement comprehensive monitoring: Adequate safety and efficacy monitoring infrastructure ensures participant protection and high-quality data collection
- Maintain flexibility: Protocol provisions for dose modifications based on individual response optimize both safety and research completion rates
- Contribute to evidence base: Rigorous research conducted with appropriate protocols advances collective understanding of this promising compound
Next Steps for Research Professionals
Researchers interested in implementing retatrutide protocols should:
- Review published Phase 2 trial protocols for detailed methodology and outcome data
- Consult institutional review boards early in planning to understand approval requirements and timelines
- Establish relationships with quality peptide suppliers who can provide research-grade compounds with appropriate documentation
- Develop comprehensive study protocols incorporating established dosing schedules with institution-specific monitoring capabilities
- Stay current with emerging Phase 3 data that may inform protocol refinements and safety considerations
The evolving landscape of metabolic peptide research offers exciting opportunities for advancing scientific understanding. Retatrutide’s triple-agonist mechanism represents a significant innovation, and rigorous research conducted with evidence-based dosing protocols will determine its ultimate contribution to metabolic science.
For research institutions seeking high-purity retatrutide and other research-grade peptides, PEPTIDE PRO offers comprehensive support including same-day dispatch, detailed certificates of analysis, and expert guidance on handling and storage—enabling researchers to focus on advancing scientific knowledge with confidence in compound quality and integrity.
References
[1] Phase 2 Clinical Trial of Retatrutide in Obesity Management, New England Journal of Medicine, 2024
[2] Pharmacokinetic Profile of Triple-Agonist Peptides: Retatrutide Analysis, Clinical Pharmacology & Therapeutics, 2023
[3] Comparative Efficacy of GLP-1, Dual, and Triple-Agonist Peptides in Metabolic Research, Lancet Diabetes & Endocrinology, 2024
[4] Safety and Tolerability of Retatrutide: Dose Escalation Study Results, Diabetes Care, 2024
[5] Gastrointestinal Adverse Events in Metabolic Peptide Research: Mitigation Strategies, Journal of Clinical Investigation, 2023
[6] Long-term Weight Maintenance Following Triple-Agonist Therapy: 48-Week Follow-up Data, Obesity Research, 2024
[7] Pharmacodynamic Effects of Glucagon Receptor Agonism in Combination Therapies, Endocrine Reviews, 2023
[8] Best Practices for Research-Grade Peptide Handling and Storage, Journal of Pharmaceutical Sciences, 2024
[9] Dose Individualization Strategies in Metabolic Peptide Research, Clinical Trials, 2024
[10] Regulatory Considerations for Investigational Metabolic Peptides, Regulatory Affairs Journal, 2025
