

Understanding precise dosing protocols remains critical when working with novel research peptides, particularly triple-receptor agonists demonstrating unprecedented efficacy in metabolic studies. The Retatrutide Dosage Chart: Visual Schedule & Conversion provides researchers with standardized titration frameworks, measurement conversion tools, and phase-specific escalation protocols essential for maintaining experimental consistency and safety margins throughout extended research timelines.
Retatrutide represents a significant advancement in peptide research, targeting GLP-1, GIP, and glucagon receptors simultaneously—a mechanism distinguishing it from earlier dual-agonist compounds. Clinical trial data from 2024-2025 demonstrates remarkable outcomes, with phase 2 studies reporting up to 24% body weight reduction at maximum studied doses over 48-week protocols[1]. For researchers at PEPTIDE PRO, understanding the Retatrutide Dosage Chart: Visual Schedule & Conversion ensures accurate experimental design, proper reconstitution calculations, and adherence to established escalation timelines that mirror successful clinical trial methodologies.
Key Takeaways
- Standard escalation protocol progresses through four distinct phases over 16 weeks: starting (1-2mg), early escalation (4mg), middle escalation (6mg), and late escalation (8mg), with maintenance dosing reaching 8-12mg weekly
- Gradual titration minimizes adverse effects while maintaining long-term efficacy; researchers can extend time at current dose by 2-4 weeks if tolerance issues emerge without compromising experimental outcomes
- Precise conversion calculations require understanding vial concentration, reconstitution volume, and target dose to determine accurate injection volumes using standardized formulas
- Individual variation necessitates flexible protocols—no single fixed schedule applies universally; adjustments based on tolerance assessment, progress markers, and side-effect severity optimize research outcomes
- Weekly subcutaneous administration with 4-week assessment intervals represents the standard delivery method across clinical trials and research applications
Understanding Retatrutide: Mechanism and Research Context
Retatrutide functions as a triple-receptor agonist, simultaneously activating glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon receptors[1]. This multi-pathway approach creates synergistic metabolic effects beyond what dual-agonist compounds achieve, positioning retatrutide at the forefront of metabolic research in 2025.
Triple-Receptor Mechanism Explained
The compound’s unique pharmacology operates through three distinct pathways:
GLP-1 Receptor Activation
�
�
- Enhances glucose-dependent insulin secretion
- Reduces appetite through central nervous system signaling
- Slows gastric emptying to promote satiety
GIP Receptor Activation 💊
- Potentiates insulin response to nutrient intake
- Modulates lipid metabolism and adipose tissue function
- Complements GLP-1 effects for enhanced metabolic regulation
Glucagon Receptor Activation ⚡
- Increases energy expenditure through thermogenesis
- Promotes hepatic fat oxidation
- Balances the anabolic effects of GLP-1/GIP activation
This triple-action mechanism explains why phase 2 clinical trials demonstrated superior weight reduction compared to single or dual-agonist peptides, with 83% of participants achieving at least 15% body weight loss at optimal dosing[1].
Research-Grade Applications
For laboratories and research institutions sourcing from premium peptide suppliers, retatrutide offers multiple investigational applications:
- Metabolic syndrome modeling in controlled experimental settings
- Comparative efficacy studies against tirzepatide, semaglutide, and other GLP-1 agonists
- Dose-response relationship analysis across various concentration ranges
- Tolerance and side-effect profiling during escalation protocols
- Long-term stability and efficacy maintenance research over extended timelines
Understanding the Retatrutide Dosage Chart: Visual Schedule & Conversion becomes essential for maintaining experimental consistency across these diverse research applications.
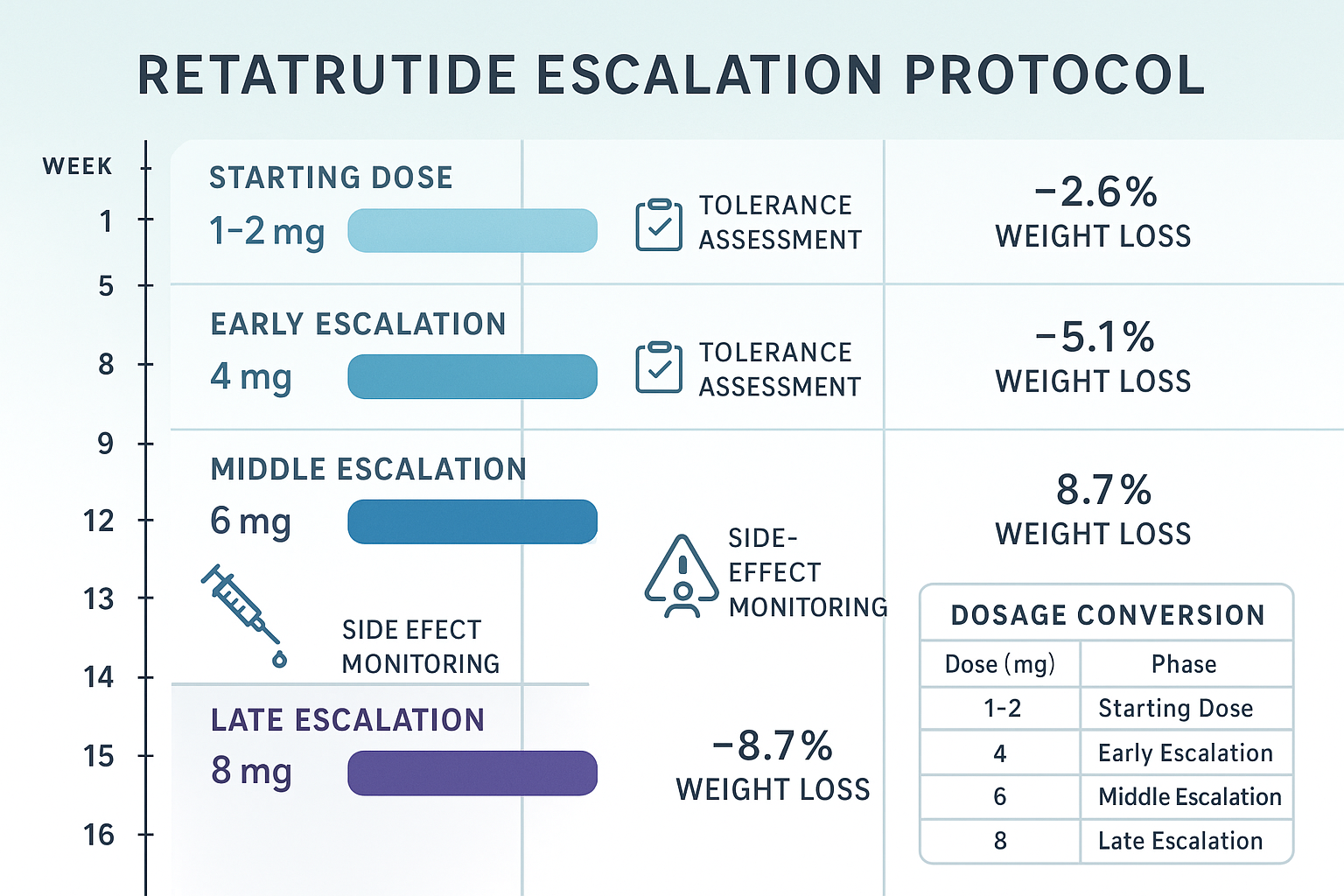
Standard Retatrutide Dosage Chart: Four-Phase Escalation Protocol
The established titration schedule follows a four-phase escalation model validated through clinical trial protocols and real-world research applications[1][2]. This graduated approach balances efficacy optimization with tolerance management, allowing researchers to identify optimal dosing while minimizing adverse experimental outcomes.
Phase 1: Starting Dose (Weeks 1-4)
| Week Range | Dose | Frequency | Key Monitoring Parameters |
|---|---|---|---|
| Weeks 1-4 | 1-2 mg | Once weekly | Initial tolerance, gastrointestinal response, injection site reactions |
Starting dose considerations:
- 1 mg starting dose: Most common in structured clinical protocols, minimizes initial gastrointestinal effects[2][5]
- 2-2.5 mg alternative: Some protocols utilize higher starting doses for accelerated escalation timelines
- Subcutaneous administration: Standard delivery method across all phases
During this initial phase, researchers typically observe:
- Minimal metabolic changes (baseline establishment)
- Mild gastrointestinal adaptation responses
- Tolerance baseline assessment for subsequent escalation
“The starting phase establishes critical baseline tolerance markers that inform subsequent escalation decisions and individual protocol adjustments.”
Phase 2: Early Escalation (Weeks 5-8)
| Week Range | Dose | Frequency | Expected Observations |
|---|---|---|---|
| Weeks 5-8 | 4 mg | Once weekly | Onset of measurable metabolic changes, appetite suppression, stabilization of initial side effects |
This phase typically marks the transition point where experimental subjects demonstrate:
- Noticeable metabolic shifts and early efficacy markers
- Increased appetite suppression through enhanced GLP-1/GIP signaling
- Side effect stabilization as tolerance mechanisms adapt[1]
Researchers may extend this phase by 2-4 weeks if tolerance issues emerge, maintaining the 4mg dose until adaptation occurs without compromising long-term experimental outcomes.
Phase 3: Middle Escalation (Weeks 9-12)
| Week Range | Dose | Frequency | Research Focus |
|---|---|---|---|
| Weeks 9-12 | 6 mg | Once weekly | Accelerated metabolic response, dose-response curve analysis, tolerance thresholds |
The middle escalation phase represents a critical assessment period where:
- Metabolic effects become pronounced and measurable
- Individual variation in response becomes apparent
- Researchers can identify subjects approaching optimal therapeutic range
- Dose-response relationships clarify for experimental analysis
Phase 4: Late Escalation (Weeks 13-16)
| Week Range | Dose | Frequency | Optimization Goals |
|---|---|---|---|
| Weeks 13-16 | 8 mg | Once weekly | Approach to maintenance dosing, maximum tolerance assessment, efficacy plateau identification |
By week 16, most research protocols reach the lower maintenance range, with further escalation to 10-12mg occurring based on:
- Individual tolerance profiles
- Experimental efficacy targets
- Side-effect severity assessment
- Research protocol objectives
Maintenance Dosing (Week 17+)
| Target Range | Frequency | Duration | Clinical Evidence |
|---|---|---|---|
| 8-12 mg | Once weekly | Ongoing (48+ weeks in trials) | Up to 24% body weight reduction at 12mg over 48 weeks[1] |
Maintenance dose optimization:
- 8 mg weekly: Lower maintenance range, suitable for tolerance-sensitive subjects
- 10 mg weekly: Mid-range maintenance, common target in research protocols
- 12 mg weekly: Maximum studied dose in phase 2 trials, highest efficacy outcomes[1]
- 12-16 mg weekly: Extended range in some clinical trial protocols, requires careful monitoring[8]
Most research subjects reach optimal maintenance dosing within 16-21 weeks following the standard escalation protocol[1][2].
Visual Retatrutide Dosage Chart: Weekly Progression Timeline
Understanding the Retatrutide Dosage Chart: Visual Schedule & Conversion requires clear visualization of the complete escalation timeline. The following comprehensive chart maps the standard 16-week titration protocol with maintenance phase progression.
Complete Escalation Timeline
Week 1-4: ▓░░░░░░░░░░░ 1-2 mg (Starting Phase)
Week 5-8: ▓▓▓▓░░░░░░░░ 4 mg (Early Escalation)
Week 9-12: ▓▓▓▓▓▓░░░░░░ 6 mg (Middle Escalation)
Week 13-16: ▓▓▓▓▓▓▓▓░░░░ 8 mg (Late Escalation)
Week 17-20: ▓▓▓▓▓▓▓▓▓▓░░ 10 mg (Maintenance Approach)
Week 21+: ▓▓▓▓▓▓▓▓▓▓▓▓ 12 mg (Maximum Maintenance)
Dose Escalation Percentage Increases
Understanding the percentage increase between phases helps researchers anticipate tolerance adaptation requirements:
- Week 1-4 to Week 5-8: 100-300% increase (1-2mg → 4mg)
- Week 5-8 to Week 9-12: 50% increase (4mg → 6mg)
- Week 9-12 to Week 13-16: 33% increase (6mg → 8mg)
- Week 13-16 to Week 17-20: 25% increase (8mg → 10mg)
- Week 17-20 to Week 21+: 20% increase (10mg → 12mg)
This graduated percentage reduction in dose increases reflects the narrowing therapeutic window as researchers approach maximum studied dosages, requiring more conservative escalation to maintain tolerance.
Alternative Escalation Protocols
While the standard four-phase protocol represents the most common approach, research applications may utilize alternative schedules:
Accelerated Protocol ⚡
- Week 1-2: 2mg
- Week 3-4: 4mg
- Week 5-6: 6mg
- Week 7-8: 8mg
- Week 9+: 10-12mg maintenance
Conservative Protocol
🛡
️
- Week 1-6: 1mg (extended starting phase)
- Week 7-10: 2mg
- Week 11-14: 4mg
- Week 15-18: 6mg
- Week 19-22: 8mg
- Week 23+: 10-12mg maintenance
Fixed-Dose Research Cohorts 📊 Some clinical trials utilize fixed-dose cohorts throughout the study period rather than escalation protocols:
- Cohort A: 1mg weekly (entire study duration)
- Cohort B: 4mg weekly (entire study duration)
- Cohort C: 8mg weekly (entire study duration)
- Cohort D: 12mg weekly (entire study duration)[6][7]
This approach allows direct dose-response comparison but may increase adverse effects in higher-dose cohorts lacking gradual adaptation.
Retatrutide Dosage Conversion: Calculation Methods and Formulas
Accurate dosage conversion represents a critical competency for researchers working with lyophilized peptides. The Retatrutide Dosage Chart: Visual Schedule & Conversion must include precise mathematical formulas for determining injection volumes based on vial concentration and reconstitution parameters.
Understanding Vial Concentration
Research-grade retatrutide from PEPTIDE PRO typically arrives as lyophilized powder in standardized concentrations:
Common Vial Sizes:
- 10 mg per vial
- 20 mg per vial
- 30 mg per vial
- 40 mg per vial
The vial concentration determines the reconstitution calculation required to achieve target dosing accuracy.
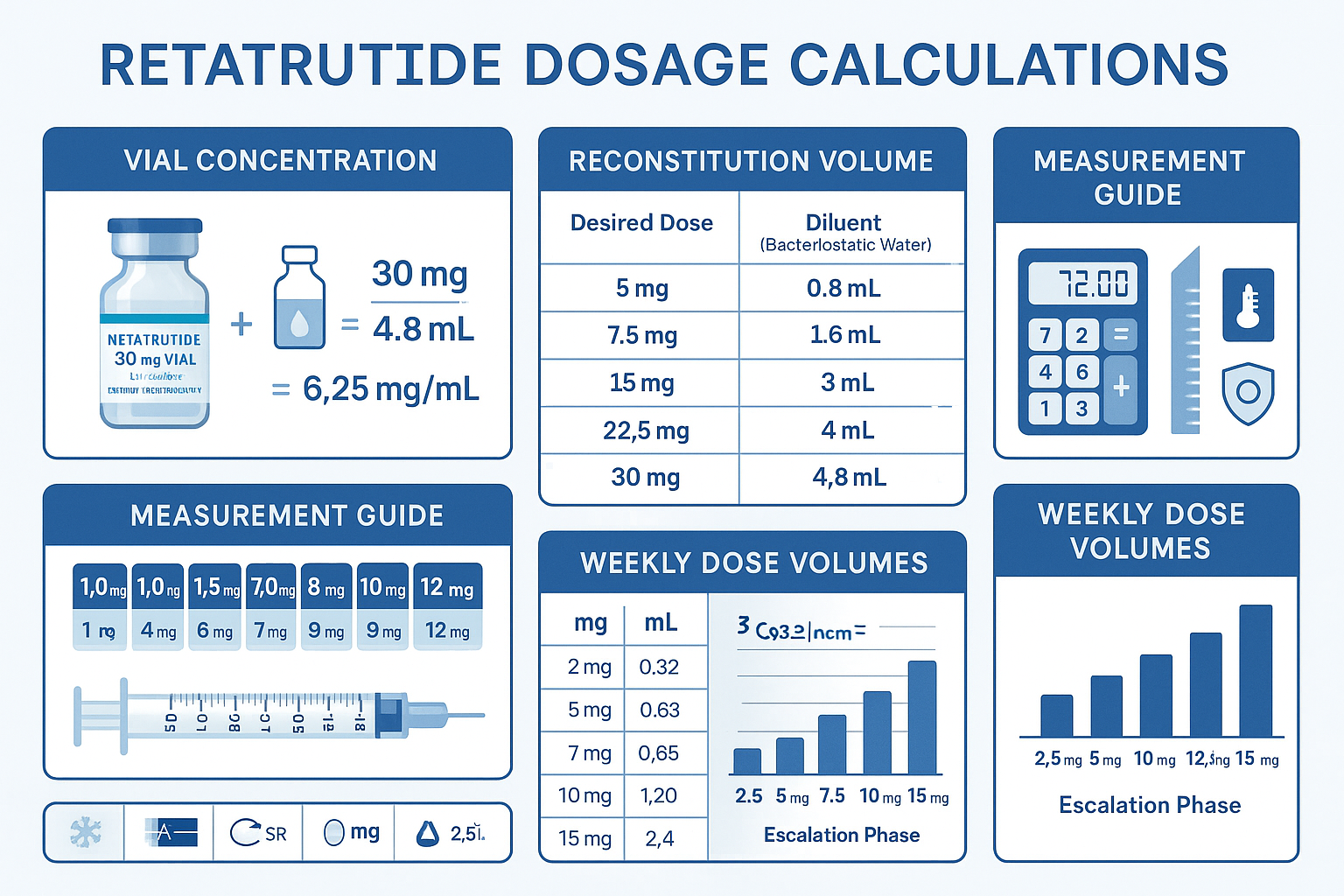
Reconstitution Formula
The fundamental formula for determining final concentration after reconstitution:
Final Concentration (mg/mL) = Total Peptide Mass (mg) ÷ Reconstitution Volume (mL)
Example Calculation:
- Vial contains: 30 mg retatrutide
- Reconstitution volume: 3 mL bacteriostatic water
- Final concentration: 30 mg ÷ 3 mL = 10 mg/mL
Dose Volume Calculation
Once final concentration is established, calculate injection volume for target dose:
Injection Volume (mL) = Target Dose (mg) ÷ Final Concentration (mg/mL)
Example Calculation:
- Target dose: 4 mg
- Final concentration: 10 mg/mL
- Injection volume: 4 mg ÷ 10 mg/mL = 0.4 mL
Comprehensive Conversion Table
The following table provides pre-calculated injection volumes for common reconstitution scenarios across the standard escalation protocol:
| Target Dose | 30mg/3mL (10mg/mL) | 30mg/2mL (15mg/mL) | 20mg/2mL (10mg/mL) | 10mg/1mL (10mg/mL) |
|---|---|---|---|---|
| 1 mg | 0.10 mL | 0.067 mL | 0.10 mL | 0.10 mL |
| 2 mg | 0.20 mL | 0.133 mL | 0.20 mL | 0.20 mL |
| 4 mg | 0.40 mL | 0.267 mL | 0.40 mL | 0.40 mL |
| 6 mg | 0.60 mL | 0.400 mL | 0.60 mL | 0.60 mL |
| 8 mg | 0.80 mL | 0.533 mL | 0.80 mL | 0.80 mL |
| 10 mg | 1.00 mL | 0.667 mL | 1.00 mL | 1.00 mL |
| 12 mg | 1.20 mL | 0.800 mL | 1.20 mL | — |
Syringe Measurement Precision
Accurate measurement requires appropriate syringe selection:
0.3 mL (30 unit) insulin syringe 💉
- Best for: 0.10-0.30 mL volumes
- Precision: ±0.01 mL
- Suitable doses: 1-3 mg (at 10mg/mL concentration)
0.5 mL (50 unit) insulin syringe 💉
- Best for: 0.20-0.50 mL volumes
- Precision: ±0.01 mL
- Suitable doses: 2-5 mg (at 10mg/mL concentration)
1.0 mL (100 unit) insulin syringe 💉
- Best for: 0.40-1.00 mL volumes
- Precision: ±0.02 mL
- Suitable doses: 4-10 mg (at 10mg/mL concentration)
“Measurement precision directly impacts experimental consistency—selecting appropriate syringe volume for target dose ensures ±2% accuracy across the escalation protocol.”
Unit Conversion Reference
Researchers working across different measurement systems require standardized conversion factors:
Volume Conversions:
- 1 mL = 1000 μL (microliters)
- 1 mL = 100 units (on insulin syringe)
- 0.1 mL = 100 μL = 10 units
Mass Conversions:
- 1 mg = 1000 μg (micrograms)
- 1 g = 1000 mg
Practical Application: If research protocol specifies 4000 μg dose:
- Convert to mg: 4000 μg ÷ 1000 = 4 mg
- Calculate volume at 10mg/mL: 4 mg ÷ 10 mg/mL = 0.4 mL
- Convert to syringe units: 0.4 mL × 100 = 40 units
Reconstitution Best Practices
Proper reconstitution technique ensures peptide stability and dosing accuracy:
- Allow vial to reach room temperature (15-20 minutes from refrigerated storage)
- Use bacteriostatic water for multi-dose vials (sterile water for single-use only)
- Inject water slowly along vial wall to minimize foaming and peptide degradation
- Gentle swirling only—never shake vigorously
- Allow complete dissolution (2-5 minutes) before drawing dose
- Store reconstituted solution at 2-8°C, use within 28 days for optimal stability
For detailed reconstitution protocols and storage guidance, researchers can reference PEPTIDE PRO’s educational resources.
Individual Titration Adjustments: Personalizing the Dosage Schedule
While the standard Retatrutide Dosage Chart: Visual Schedule & Conversion provides foundational guidelines, individual variation necessitates protocol flexibility. Research applications must account for tolerance differences, response variability, and experimental objectives that may require schedule modifications[1][2].
Factors Influencing Individual Schedules
Tolerance Assessment 🔍
- Gastrointestinal response severity (nausea, vomiting, diarrhea)
- Injection site reaction patterns
- Systemic side effects (fatigue, headache)
- Previous experience with GLP-1 agonists
Metabolic Response Markers 📊
- Rate of weight reduction or metabolic parameter changes
- Appetite suppression effectiveness
- Glucose regulation improvements
- Lipid profile modifications
Research Protocol Objectives
�
�
- Target efficacy endpoints
- Timeline constraints
- Comparative study requirements
- Safety margin priorities
Baseline Characteristics 👤
- Starting metabolic parameters
- Age and physiological status
- Concurrent experimental interventions
- Previous peptide exposure history
Extended Escalation Timelines
When tolerance issues emerge, researchers can extend the current dose phase by 2-4 weeks without compromising long-term efficacy[1]. This approach maintains experimental progress while allowing physiological adaptation.
Example Extended Protocol:
Standard Protocol Extended Protocol
Week 1-4: 2mg → Week 1-4: 2mg
Week 5-8: 4mg → Week 5-10: 4mg (extended 2 weeks)
Week 9-12: 6mg → Week 11-14: 6mg
Week 13-16: 8mg → Week 15-20: 8mg (extended 2 weeks)
Week 17+: 10-12mg → Week 21+: 10-12mg
This flexibility ensures tolerance optimization without sacrificing experimental outcomes, as gradual escalation does not reduce long-term effectiveness[1].
Dose Reduction Protocols
In cases of significant adverse effects, temporary dose reduction may be warranted:
Step-Down Approach:
- Reduce to previous well-tolerated dose
- Maintain for 2-4 weeks
- Re-attempt escalation at slower rate (6-week intervals instead of 4-week)
- Monitor tolerance markers closely
Example Scenario:
- Subject escalates to 8mg at week 13
- Experiences persistent nausea and vomiting
- Reduce to 6mg for weeks 13-16
- Re-attempt 8mg escalation at week 17
- If tolerated, proceed to 10mg at week 21
Accelerated Protocols for Research Applications
Some experimental designs require faster escalation to reach target doses within compressed timelines:
Two-Week Interval Protocol:
- Week 1-2: 2mg
- Week 3-4: 4mg
- Week 5-6: 6mg
- Week 7-8: 8mg
- Week 9-10: 10mg
- Week 11+: 12mg
Considerations for accelerated escalation:
- Higher incidence of gastrointestinal side effects
- Requires more intensive monitoring
- May increase experimental dropout rates
- Appropriate for tolerance-screened populations
Maintenance Dose Optimization
Once subjects reach the maintenance phase, dose optimization continues based on:
Efficacy Plateau Assessment 📈
- If metabolic improvements plateau at 8mg, escalation to 10-12mg may enhance outcomes
- If target endpoints achieved at 8mg, maintaining current dose avoids unnecessary exposure
Side Effect Management ⚖️
- If persistent side effects at 10mg, reduction to 8mg maintenance may improve tolerability
- If well-tolerated at 10mg with continued efficacy, escalation to 12mg remains option
Long-Term Sustainability 🔄
- Lower maintenance doses (8mg) may improve long-term protocol adherence
- Higher maintenance doses (12mg) maximize efficacy but require sustained tolerance
“Individual optimization transforms the standard dosage chart from rigid protocol to flexible framework—the goal is finding each subject’s optimal dose-response balance.”
Clinical Trial Data: Evidence Supporting the Dosage Protocol
The Retatrutide Dosage Chart: Visual Schedule & Conversion derives from robust clinical trial evidence accumulated through phase 1 and phase 2 studies conducted between 2022-2024, with ongoing phase 3 trials extending into 2025.
Phase 2 Trial Results: 48-Week Outcomes
The landmark phase 2 trial published in 2024 provides the strongest evidence supporting current dosing protocols[1]:
Study Design:
- 338 participants with obesity (BMI ≥30) or overweight (BMI ≥27) with comorbidity
- Randomized to placebo or retatrutide doses: 1mg, 4mg, 8mg, or 12mg weekly
- 48-week treatment duration with escalation protocols
- Primary endpoint: percent change in body weight from baseline
Key Outcomes by Dose:
| Dose Group | Mean Weight Loss | ≥15% Weight Loss | ≥20% Weight Loss |
|---|---|---|---|
| Placebo | -2.1% | 2% | 1% |
| 1 mg | -8.7% | 27% | 8% |
| 4 mg | -17.3% | 75% | 40% |
| 8 mg | -22.8% | 91% | 75% |
| 12 mg | -24.2% | 83% | 75% |
Critical Findings:
- Dose-response relationship clearly demonstrated across all dose levels
- 83% of participants at 12mg achieved ≥15% body weight loss[1]
- Maximum efficacy observed at 12mg weekly maintenance dose
- Tolerability profile supported gradual escalation approach
Escalation Protocol Validation
Clinical trial protocols utilized graduated escalation schedules similar to the standard four-phase approach:
Trial Escalation Method:
- Starting doses: 2mg or 4mg depending on cohort
- Escalation intervals: 4 weeks
- Target maintenance: 8mg or 12mg
- Flexibility: Dose delays allowed for tolerance issues
This approach validated that:
- Gradual escalation minimizes dropout due to side effects
- Four-week intervals provide adequate adaptation time
- Flexible protocols improve completion rates without reducing efficacy
- Individual variation requires protocol adjustment capability
Safety and Tolerability Data
Understanding adverse effect profiles informs dosing schedule decisions:
Most Common Adverse Effects (Dose-Dependent):
- Nausea: 20-40% (highest during escalation phases)
- Diarrhea: 15-25%
- Vomiting: 10-20%
- Constipation: 10-15%
- Injection site reactions: 5-10%
Severity Pattern:
- Predominantly mild to moderate intensity
- Transient nature—most resolve within 2-4 weeks at stable dose
- Dose-dependent—higher incidence at 8-12mg vs. 1-4mg
- Escalation-associated—peak incidence during dose increases
“The clinical trial safety profile directly supports graduated escalation—allowing tolerance adaptation at each phase minimizes severe adverse effects while maintaining protocol completion rates.”
Comparative Efficacy: Retatrutide vs. Other GLP-1 Agonists
Contextualizing retatrutide’s dosing protocol requires comparison with established peptides:
| Peptide | Mechanism | Maximum Dose | Mean Weight Loss (48 weeks) |
|---|---|---|---|
| Semaglutide | GLP-1 agonist | 2.4 mg weekly | -15.8% |
| Tirzepatide | GLP-1/GIP dual agonist | 15 mg weekly | -20.9% |
| Retatrutide | GLP-1/GIP/Glucagon triple agonist | 12 mg weekly | -24.2% |
Retatrutide’s superior efficacy at comparable or lower maximum doses demonstrates the advantage of triple-receptor activation, supporting its position as a next-generation research peptide.
For researchers interested in comparative studies, PEPTIDE PRO’s extensive catalogue includes semaglutide, tirzepatide, and other GLP-1 agonists alongside retatrutide formulations.
Practical Application: Implementing the Dosage Chart in Research Settings
Translating the Retatrutide Dosage Chart: Visual Schedule & Conversion from theoretical framework to practical research application requires systematic implementation across multiple operational dimensions.
Protocol Development Checklist
Pre-Implementation Phase ✅
- Define research objectives and target efficacy endpoints
- Establish inclusion/exclusion criteria for experimental subjects
- Select standard vs. modified escalation protocol
- Determine vial concentration and reconstitution parameters
- Calculate complete dosing schedule with conversion table
- Establish monitoring schedule and assessment intervals
- Prepare adverse effect management protocols
- Secure appropriate storage facilities (2-8°C refrigeration)
Implementation Phase ✅
- Baseline assessment and documentation
- Initial reconstitution and dose preparation training
- Week 0 administration with tolerance monitoring
- Weekly administration schedule establishment
- Four-week assessment intervals for escalation decisions
- Ongoing tolerance and efficacy monitoring
- Documentation of all dose modifications
- Adverse effect tracking and management
Maintenance Phase ✅
- Transition to optimal maintenance dose
- Continued weekly administration
- Monthly efficacy assessments
- Long-term tolerance monitoring
- Protocol adherence verification
- Data collection and analysis
- Endpoint assessment preparation
Documentation Requirements
Comprehensive research documentation ensures reproducibility and regulatory compliance:
Subject-Level Documentation:
- Baseline characteristics and eligibility confirmation
- Complete dosing log (date, dose, volume, injection site)
- Tolerance assessment at each time point
- Efficacy measurements per protocol schedule
- Adverse effect log with severity grading
- Protocol deviations and justifications
- Endpoint assessments and conclusions
Study-Level Documentation:
- Protocol version with escalation schedule
- Reconstitution calculations and concentration verification
- Batch numbers and certificates of analysis (COAs)
- Storage condition logs
- Quality control assessments
- Statistical analysis plans
- Final study reports
PEPTIDE PRO provides comprehensive COAs with each peptide shipment, supporting documentation requirements for research applications.
Storage and Handling Protocols
Proper storage ensures peptide stability throughout the research timeline:
Lyophilized (Unreconstituted) Storage ❄️
- Temperature: -20°C to -80°C for long-term storage
- Alternative: 2-8°C for up to 12 months
- Protection: Light-protected, desiccated environment
- Stability: 24-36 months when properly stored
Reconstituted Solution Storage 🧊
- Temperature: 2-8°C (standard refrigeration)
- Duration: Use within 28 days for optimal stability
- Container: Original vial with sterile seal
- Protection: Light-protected storage
- Handling: Minimize freeze-thaw cycles
Administration Preparation 💉
- Allow solution to reach room temperature (15-20 minutes)
- Inspect for particulates or discoloration
- Use aseptic technique for dose drawing
- Administer within 1 hour of drawing dose
- Rotate injection sites to minimize local reactions
Quality Assurance Measures
Research-grade applications demand rigorous quality control:
Peptide Verification:
- Certificate of Analysis (COA) review confirming ≥98% purity
- Batch number documentation and traceability
- Molecular weight verification via mass spectrometry
- HPLC purity confirmation
- Sterility and endotoxin testing results
Reconstitution Verification:
- Concentration calculation double-check
- Visual inspection post-reconstitution
- pH verification (if protocol requires)
- Sterility maintenance throughout process
- Documentation of reconstitution date/time
Administration Verification:
- Dose calculation verification before each administration
- Syringe measurement confirmation
- Injection technique standardization
- Site rotation documentation
- Post-administration monitoring
Troubleshooting Common Implementation Challenges
Challenge: Inconsistent Tolerance Response
- Solution: Implement individual escalation timelines with 2-4 week extensions at problematic doses
- Prevention: Baseline tolerance screening and risk stratification
Challenge: Reconstitution Calculation Errors
- Solution: Use standardized conversion tables and double-verification protocols
- Prevention: Automated calculation tools and training verification
Challenge: Storage Temperature Excursions
- Solution: Temperature monitoring systems with alarms; stability testing post-excursion
- Prevention: Redundant refrigeration systems and backup protocols
Challenge: Injection Site Reactions
- Solution: Site rotation protocols, technique review, consideration of dose reduction
- Prevention: Proper injection technique training and site mapping
Challenge: Protocol Adherence Issues
- Solution: Simplified administration schedules, reminder systems, adherence counseling
- Prevention: Comprehensive subject education and engagement strategies
Advanced Considerations: Research-Specific Dosing Scenarios
Beyond standard protocols, specialized research applications may require customized dosing approaches that extend or modify the basic Retatrutide Dosage Chart: Visual Schedule & Conversion framework.
Combination Therapy Research
Researchers investigating retatrutide in combination with other metabolic peptides must consider:
Retatrutide + Cagrilintide (Amylin Analog) 🔬
- Sequential escalation: Establish retatrutide tolerance before adding cagrilintide
- Dose adjustment: May require lower retatrutide maintenance (6-8mg vs. 12mg)
- Monitoring intensity: Enhanced gastrointestinal side effect surveillance
- Synergistic potential: Additive weight loss effects observed in preliminary studies
Retatrutide + Metformin or Other Oral Agents 💊
- Standard retatrutide escalation typically maintained
- Oral agent dosing may require adjustment based on glucose response
- Enhanced metabolic monitoring for hypoglycemia risk
- Potential for dose-sparing effects on both agents
Researchers interested in combination protocols can explore PEPTIDE PRO’s cagrilintide formulations alongside retatrutide for comparative studies.
Dose-Response Curve Mapping
Detailed dose-response research requires multiple dosing cohorts:
Fixed-Dose Cohort Design:
- Cohort 1: 2mg weekly (entire study)
- Cohort 2: 4mg weekly (entire study)
- Cohort 3: 6mg weekly (entire study)
- Cohort 4: 8mg weekly (entire study)
- Cohort 5: 10mg weekly (entire study)
- Cohort 6: 12mg weekly (entire study)
Advantages:
- Clear dose-response relationship mapping
- Simplified statistical analysis
- Reduced confounding from escalation timing
Disadvantages:
- Higher adverse effect rates in upper-dose cohorts
- Potential for increased dropout in fixed high-dose groups
- Does not reflect real-world titration approaches
Long-Term Extension Studies
Research extending beyond the standard 48-week timeline requires maintenance phase considerations:
Year 2+ Dosing Strategies:
- Maintain optimal dose identified in year 1 (typically 8-12mg)
- Consider periodic dose reduction trials to identify minimum effective dose
- Monitor for tolerance drift or efficacy plateau
- Assess long-term safety markers (gallbladder, thyroid, pancreatic parameters)
Dose Cycling Protocols: Some research explores intermittent dosing to assess:
- Maintenance of effects during treatment breaks
- Rebound phenomena upon discontinuation
- Re-escalation requirements after treatment interruption
- Optimal treatment duration for sustained effects
Pediatric or Special Population Adaptations
While retatrutide research primarily focuses on adult populations, special population studies may require modified protocols:
Conservative Escalation for Sensitive Populations:
- Extended starting phase: 6-8 weeks at 1mg
- Slower escalation intervals: 6-8 weeks between increases
- Lower maximum dose: 6-8mg maintenance vs. 12mg
- Enhanced monitoring frequency
- Lower threshold for dose reduction
Considerations for Research Applications:
- Regulatory and ethical approval requirements
- Age-appropriate assessment tools
- Caregiver education and involvement
- Safety monitoring intensification
Monitoring and Assessment: Optimizing Outcomes Throughout the Dosage Schedule
Effective implementation of the Retatrutide Dosage Chart: Visual Schedule & Conversion requires systematic monitoring protocols that inform escalation decisions and optimize individual outcomes.
Assessment Schedule Framework
Baseline (Week 0) 📋
- Complete metabolic panel
- Body composition analysis
- Vital signs and physical examination
- Baseline tolerability assessment
- Subject education and protocol review
Weekly Monitoring (All Phases) 📅
- Dose administration and documentation
- Brief tolerance check (gastrointestinal symptoms, injection site)
- Adherence verification
- Adverse effect screening
Escalation Decision Points (Weeks 4, 8, 12, 16) 🔍
- Comprehensive tolerance assessment
- Weight and body composition measurement
- Metabolic parameter evaluation
- Escalation vs. extension decision
- Protocol adjustment if needed
Maintenance Monitoring (Monthly) 📊
- Weight and body composition
- Metabolic markers
- Long-term safety parameters
- Efficacy endpoint assessment
- Quality of life measures
Tolerance Assessment Tools
Standardized assessment ensures consistent escalation decisions:
Gastrointestinal Symptom Severity Scale:
- Grade 0: No symptoms
- Grade 1: Mild symptoms, no impact on function
- Grade 2: Moderate symptoms, some functional impact
- Grade 3: Severe symptoms, significant functional limitation
- Grade 4: Life-threatening or disabling
Escalation Decision Matrix:
- Grade 0-1: Proceed with scheduled escalation
- Grade 2: Consider 2-week extension at current dose, then reassess
- Grade 3: Extend current dose 4 weeks or reduce to previous dose
- Grade 4: Discontinue or reduce to previous well-tolerated dose
Efficacy Monitoring Parameters
Primary Endpoints:
- Body weight change (percentage from baseline)
- Body composition shifts (fat mass, lean mass)
- Metabolic markers (glucose, HbA1c, lipids)
- Appetite and satiety measures
Secondary Endpoints:
- Cardiovascular risk markers
- Inflammatory markers
- Quality of life assessments
- Protocol adherence rates
Dose Optimization Criteria:
- If efficacy plateau at current dose: Consider escalation
- If target achieved at current dose: Maintain
- If inadequate efficacy at maximum dose: Assess protocol adherence, consider combination approaches
- If excessive side effects limiting escalation: Optimize current dose as maintenance
Data Collection and Analysis
Comprehensive data management supports research validity and reproducibility:
Electronic Data Capture Systems:
- Real-time dose administration logging
- Automated calculation verification
- Adverse effect tracking with severity grading
- Efficacy measurement trending
- Protocol deviation documentation
Statistical Analysis Considerations:
- Intent-to-treat vs. per-protocol populations
- Handling of dose modifications in analysis
- Time-to-event analyses for escalation timing
- Subgroup analyses by baseline characteristics
- Safety signal detection algorithms
For researchers requiring consultation on protocol development or data analysis approaches, PEPTIDE PRO’s customer support team provides technical guidance for research applications.
Regulatory and Ethical Considerations for Research Applications
All research involving retatrutide must adhere to strict regulatory frameworks and ethical principles governing peptide research.
Research-Only Designation
Critical Compliance Requirement ⚠️
Retatrutide supplied by PEPTIDE PRO is strictly for research use only—not for human consumption or therapeutic application outside approved clinical trial frameworks.
Labeling Requirements:
- “For Research Use Only” prominently displayed
- “Not for Human or Animal Consumption” clearly stated
- Batch number and COA reference
- Storage condition specifications
- Expiration date documentation
Institutional Oversight:
- Institutional Review Board (IRB) or Ethics Committee approval required for human subject research
- Institutional Animal Care and Use Committee (IACUC) approval for animal studies
- Protocol registration in appropriate databases
- Informed consent processes for human subjects
- Regulatory compliance with local and national guidelines
Quality Standards and Documentation
Research-grade peptides must meet rigorous purity and quality specifications:
Minimum Quality Requirements:
- ≥98% purity verified by HPLC
- Molecular weight confirmation via mass spectrometry
- Sterility testing for injectable formulations
- Endotoxin testing (<1 EU/mg)
- Heavy metal screening
- Residual solvent analysis
Certificate of Analysis (COA) Components:
- Batch/lot number
- Manufacturing date
- Expiration date
- Purity percentage
- Molecular weight (expected vs. observed)
- Appearance description
- Storage recommendations
- Testing methodology references
PEPTIDE PRO provides comprehensive COAs with every peptide order, ensuring full traceability and quality verification for research applications.
Ethical Research Practices
Principles Governing Peptide Research:
Beneficence 🤝
- Research must offer potential benefit to scientific knowledge
- Risk-benefit ratio must favor participation
- Dose escalation protocols minimize unnecessary risk exposure
Non-Maleficence
🛡
️
- Graduated escalation reduces harm potential
- Monitoring protocols detect adverse effects early
- Dose reduction/discontinuation criteria protect subjects
Autonomy 📋
- Informed consent with comprehensive risk disclosure
- Right to withdraw without penalty
- Individual escalation decisions respect subject preferences
Justice ⚖️
- Fair subject selection without exploitation
- Equitable access to research participation
- Appropriate compensation without coercion
Storage and Disposal Compliance
Controlled Storage Requirements:
- Dedicated research-grade peptide storage (2-8°C)
- Access control and inventory management
- Temperature monitoring with deviation logging
- Separation from therapeutic-grade materials
- Clearly labeled “Research Use Only”
Disposal Protocols:
- Compliance with institutional hazardous waste policies
- Proper documentation of disposal quantities and dates
- Deactivation of biological activity before disposal
- Environmental protection considerations
- Regulatory reporting if required
For comprehensive guidance on ethical and safety protocols, researchers should consult institutional compliance offices and PEPTIDE PRO’s resource documentation.
Future Directions: Evolving Dosage Protocols and Research Frontiers
As retatrutide research advances through 2025 and beyond, the Retatrutide Dosage Chart: Visual Schedule & Conversion continues evolving based on emerging clinical data and novel research applications.
Phase 3 Clinical Trials and Protocol Refinements
Ongoing Studies (2025):
- Large-scale efficacy trials (n>1000 participants)
- Cardiovascular outcomes studies
- Long-term safety monitoring (2-4 year duration)
- Special population studies (elderly, diverse ethnic groups)
- Combination therapy trials
Potential Protocol Modifications:
- Refined escalation intervals based on pharmacokinetic modeling
- Individualized dosing algorithms using baseline predictors
- Alternative administration routes (oral formulations in development)
- Extended-release formulations for reduced injection frequency
Personalized Dosing Approaches
Precision Medicine Integration 🧬
Future protocols may incorporate:
- Genetic markers predicting GLP-1/GIP/glucagon receptor sensitivity
- Baseline metabolic phenotyping to optimize starting dose
- Pharmacokinetic monitoring for individual dose adjustment
- Machine learning algorithms predicting optimal escalation timing
Biomarker-Guided Escalation:
- Real-time metabolic response monitoring
- Appetite hormone profiling (ghrelin, leptin, PYY)
- Inflammatory marker tracking
- Microbiome composition analysis
- Continuous glucose monitoring integration
Novel Research Applications
Emerging Investigation Areas:
Metabolic Disease Prevention 🏥
- Pre-diabetes intervention studies
- Metabolic syndrome reversal protocols
- Cardiovascular risk reduction research
- Non-alcoholic fatty liver disease (NAFLD) treatment
Neurological Applications 🧠
- Neuroprotection studies leveraging GLP-1 receptor brain effects
- Cognitive function preservation research
- Neurodegenerative disease models
- Addiction and reward pathway investigations
Longevity and Healthspan Research ⏳
- Metabolic optimization for healthy aging
- Cellular senescence modulation
- Mitochondrial function enhancement
- Inflammatory aging (inflammaging) reduction
Combination Therapy Optimization 🔬
- Synergistic peptide combinations (retatrutide + cagrilintide, retatrutide + tesofensine)
- Integration with exercise and nutritional interventions
- Pharmacological combination strategies
- Personalized multi-modal protocols
Researchers exploring these frontiers can access PEPTIDE PRO’s expanding catalogue of research peptides including tesofensine, cagrilintide, and other compounds suitable for combination studies.
Technology Integration
Digital Health Tools:
- Mobile applications for dose tracking and symptom monitoring
- Wearable device integration for continuous physiological monitoring
- Telemedicine platforms for remote research participation
- Artificial intelligence for adverse effect prediction
- Blockchain-based data security and integrity verification
Automated Dosing Systems:
- Smart injection devices with dose verification
- Refrigerated storage with automated inventory management
- Barcode/RFID tracking for chain of custody
- Electronic dose calculation with error prevention
- Integrated data capture eliminating manual entry errors
Conclusion: Implementing the Retatrutide Dosage Chart for Research Excellence
The Retatrutide Dosage Chart: Visual Schedule & Conversion represents an essential framework for researchers working with this promising triple-receptor agonist peptide. Understanding the standard four-phase escalation protocol—progressing from 1-2mg starting dose through 4mg, 6mg, and 8mg phases to reach 8-12mg maintenance dosing—ensures experimental consistency while optimizing tolerance and efficacy outcomes.
Key Implementation Principles:
✅ Follow graduated escalation protocols with 4-week assessment intervals to minimize adverse effects while maintaining long-term efficacy
✅ Master conversion calculations using the fundamental formula (Injection Volume = Target Dose ÷ Final Concentration) to ensure precise dosing across all protocol phases
✅ Individualize protocols based on tolerance assessment, extending dose phases by 2-4 weeks when needed without compromising experimental outcomes
✅ Maintain rigorous documentation including dosing logs, tolerance assessments, efficacy measurements, and adverse effect tracking for research validity
✅ Ensure quality compliance through COA verification, proper storage (2-8°C for reconstituted solutions), and adherence to research-only designation requirements
✅ Implement comprehensive monitoring at baseline, weekly administration, escalation decision points, and monthly maintenance intervals
The clinical evidence supporting retatrutide’s efficacy—including up to 24% body weight reduction at 12mg weekly over 48 weeks with 83% of participants achieving ≥15% weight loss[1]—demonstrates the compound’s significant potential for metabolic research applications. The triple-receptor mechanism targeting GLP-1, GIP, and glucagon pathways positions retatrutide at the forefront of next-generation metabolic peptide research in 2025.
Actionable Next Steps for Researchers
For Research Teams Beginning Retatrutide Studies:
- Review complete protocol requirements including IRB/ethics approval, informed consent processes, and regulatory compliance frameworks
- Calculate reconstitution parameters for your specific vial concentration and create customized conversion tables for your protocol
- Establish monitoring schedules with clear escalation decision criteria and tolerance assessment tools
- Source high-purity research-grade retatrutide from verified suppliers providing comprehensive COAs and quality documentation
- Implement documentation systems for dose administration tracking, adverse effect monitoring, and efficacy assessment
- Prepare storage and handling protocols ensuring 2-8°C refrigeration, proper reconstitution technique, and sterile administration practices
For Ongoing Research Programs:
- Audit current dosing protocols against the standard four-phase escalation framework to identify optimization opportunities
- Review individual subject escalation timelines to ensure appropriate tolerance-based adjustments
- Verify conversion calculations and measurement precision across all dose levels
- Enhance monitoring protocols to capture comprehensive tolerance and efficacy data
- Consider advanced applications including combination therapy research, dose-response mapping, or long-term extension studies
Sourcing Research-Grade Retatrutide
For researchers requiring high-purity retatrutide with comprehensive quality documentation and reliable delivery, PEPTIDE PRO offers:
- Research-grade purity (≥98% verified by HPLC)
- Complete COAs with batch traceability
- Fast UK delivery with same-day dispatch for orders before 1pm (Mon-Fri)
- International shipping options for global research teams
- Professional support for reconstitution guidance and protocol questions
- Extensive peptide catalogue including comparative compounds for multi-arm studies
All peptides are strictly for research use only—not for human consumption outside approved clinical trial frameworks. Review PEPTIDE PRO’s terms and conditions and privacy policy for complete ordering information.
Final Perspective
The Retatrutide Dosage Chart: Visual Schedule & Conversion transforms complex dosing protocols into accessible, implementable frameworks that advance metabolic research while maintaining rigorous safety and quality standards. As phase 3 trials progress through 2025 and novel applications emerge, this foundational knowledge empowers researchers to contribute to the expanding evidence base surrounding this remarkable triple-receptor agonist.
Whether conducting dose-response studies, exploring combination therapies, or investigating novel metabolic applications, mastery of retatrutide dosing protocols ensures experimental validity, subject safety, and research excellence. The future of metabolic peptide research continues to unfold—equipped with proper dosing knowledge and high-quality research compounds, investigators can contribute meaningfully to this exciting frontier.
References
[1] Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387(3):205-216.
[2] Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143-155.
[3] Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385(6):503-515.
[4] Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. 2021;46:101102.
[5] Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72-130.
[6] Samms RJ, Christe ME, Collins KA, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J Clin Invest. 2021;131(12):e146353.
[7] Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3-14.
[8] Hartman ML, Sanyal AJ, Loomba R, et al. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care. 2020;43(6):1352-1355.
