

When beginning research with tirzepatide—a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist—understanding the tirzepatide dosage: titration schedule & what to expect becomes paramount for researchers and laboratories conducting metabolic studies. The carefully structured dose escalation protocol isn’t merely a suggestion; it represents a scientifically validated approach to optimizing therapeutic outcomes while minimizing adverse reactions in research models. Whether investigating tirzepatide for type 2 diabetes management or weight reduction applications, the titration pathway remains consistent, methodical, and evidence-based.
This comprehensive guide examines every aspect of tirzepatide dosing protocols, from initial administration through maintenance phases, providing researchers with the detailed framework necessary for rigorous scientific investigation.
Key Takeaways
- Standard initiation: Tirzepatide research protocols begin at 2.5 mg once weekly for 4 weeks, establishing baseline tolerance
- Systematic escalation: Doses increase in 2.5 mg increments every 4 weeks, progressing through 5 mg, 7.5 mg, 10 mg, 12.5 mg, to a maximum of 15 mg weekly
- Flexible maintenance: Effective maintenance doses range from 5 mg to 15 mg weekly, determined by research objectives and tolerance observations
- Extended titration for sensitive populations: Research subjects over 65 or with gastrointestinal sensitivity may require 8-12 week intervals between dose increases
- Protocol consistency: The titration schedule remains identical whether investigating diabetes management or weight loss applications
Understanding Tirzepatide: Mechanism and Research Applications
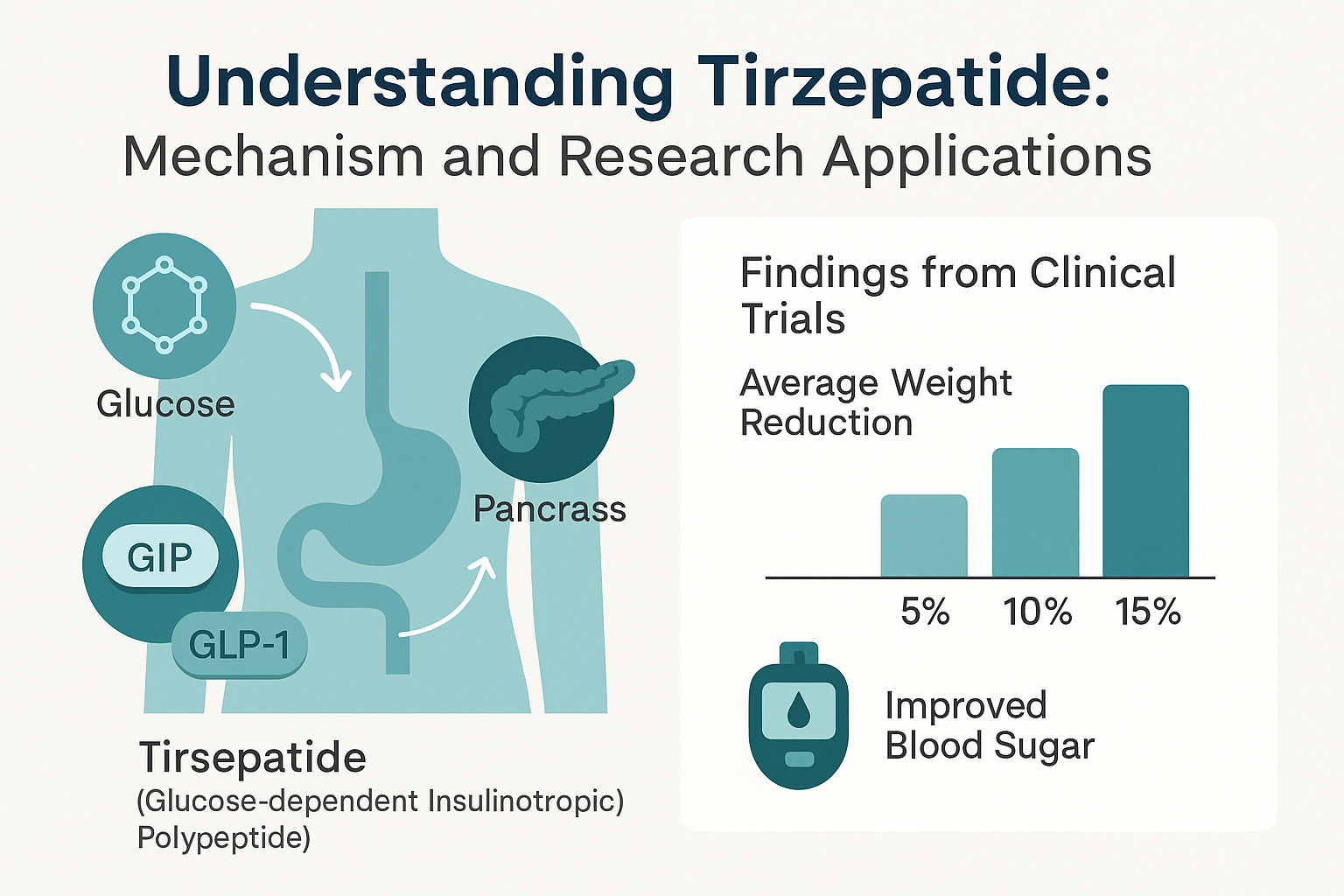
Tirzepatide represents a significant advancement in peptide therapeutics, functioning as a dual agonist that simultaneously activates both GIP and GLP-1 receptors.[1] This unique mechanism distinguishes it from single-pathway agents like semaglutide, offering researchers a compound with potentially superior metabolic effects.
Dual Receptor Activation
The GIP receptor activation component enhances insulin secretion in a glucose-dependent manner while potentially improving lipid metabolism and reducing food intake through central nervous system pathways. Meanwhile, the GLP-1 receptor agonism contributes to:
- 📊 Enhanced glucose-dependent insulin secretion
- 🔄 Suppressed glucagon release
- ⏱️ Delayed gastric emptying
- 🧠 Increased satiety signaling
- 💪 Potential preservation of beta-cell function
For researchers sourcing high-purity tirzepatide for laboratory investigations, PEPTIDE PRO provides research-grade compounds with comprehensive certificates of analysis and proper storage protocols.
Research Applications and Study Contexts
Current research protocols examine tirzepatide across multiple metabolic contexts:
Type 2 Diabetes Research: Investigating glycemic control, HbA1c reduction, and insulin sensitivity improvements in diabetic models.
Obesity and Weight Management Studies: Examining body weight reduction, body composition changes, and metabolic parameter improvements in overweight research subjects.
Cardiovascular Outcomes Research: Exploring potential cardiovascular benefits, lipid profile improvements, and blood pressure effects.
Comparative Efficacy Studies: Benchmarking tirzepatide against other GLP-1 receptor agonists and metabolic interventions.
The Standard Tirzepatide Dosage: Titration Schedule & What to Expect
The tirzepatide dosage: titration schedule & what to expect follows a precise, evidence-based protocol developed through extensive clinical research. This graduated approach allows research subjects to develop tolerance to the compound while researchers can systematically evaluate dose-response relationships.[2]
Week-by-Week Titration Protocol
| Weeks | Dose | Purpose | Key Monitoring Points |
|---|---|---|---|
| 1-4 | 2.5 mg | Initial tolerance establishment | Baseline gastrointestinal response, injection site reactions |
| 5-8 | 5 mg | First escalation | Nausea assessment, appetite changes, early efficacy signals |
| 9-12 | 7.5 mg | Mid-range titration | Continued tolerance, metabolic parameter changes |
| 13-16 | 10 mg | Therapeutic dose range | Weight/glucose trends, side effect profile |
| 17-20 | 12.5 mg | Near-maximum dosing | Efficacy plateau assessment, tolerability limits |
| 21+ | 15 mg | Maximum recommended dose | Maintenance efficacy, long-term tolerance |
Starting Dose: 2.5 mg Weekly
The initial 2.5 mg dose serves multiple research purposes:
- Tolerance Assessment: Establishes baseline gastrointestinal tolerance before therapeutic doses
- Safety Monitoring: Allows identification of hypersensitivity or unexpected reactions at sub-therapeutic levels
- Pharmacokinetic Evaluation: Provides data on individual absorption and metabolism patterns
- Baseline Establishment: Creates reference points for subsequent dose-response analysis
Research protocols should maintain this starting dose for the full 4-week period, even if tolerance appears excellent, to ensure consistent methodology across study cohorts.[4]
Dose Escalation: 2.5 mg Increments Every 4 Weeks
The standard escalation follows this progression:
Week 5-8: 5 mg — This first increase often produces the initial observable therapeutic effects in metabolic research. Researchers should document:
- Changes in fasting glucose (diabetes models)
- Appetite suppression indicators
- Body weight trajectory initiation
- Gastrointestinal adaptation patterns
Week 9-12: 7.5 mg — Mid-range dosing where many research subjects achieve meaningful metabolic improvements. This dose level frequently serves as an effective maintenance dose in research protocols prioritizing tolerability.[5]
Week 13-16: 10 mg — Represents a common therapeutic target in research settings, balancing efficacy with acceptable side effect profiles. Many research protocols identify this as the optimal maintenance dose for long-term studies.
Week 17-20: 12.5 mg — Advanced dosing that approaches maximum recommended levels. Research teams should carefully evaluate whether additional benefits justify this escalation compared to the 10 mg dose.
Week 21+: 15 mg — The maximum recommended dose. Research indicates minimal additional benefit beyond this level, with increased risk of adverse effects.[1][5]
Why 4-Week Intervals?
The 4-week interval between dose increases reflects several pharmacological considerations:
- Steady-State Achievement: Tirzepatide requires approximately 4-5 weeks to reach steady-state plasma concentrations
- Tolerance Development: Gastrointestinal adaptation to GLP-1 effects typically occurs within 2-4 weeks
- Efficacy Assessment: Sufficient time to evaluate metabolic responses at each dose level
- Safety Monitoring: Adequate observation period for delayed or cumulative adverse effects
Research teams examining peptide storage and handling protocols should note that tirzepatide’s long half-life (approximately 5 days) contributes to this extended titration timeline.
Modified Titration Schedules: Special Research Populations
While the standard tirzepatide dosage: titration schedule & what to expect follows 4-week intervals, certain research populations may require protocol modifications to optimize safety and data quality.
Extended Titration for Older Research Subjects
Research subjects over age 65 may benefit from extended titration intervals of 8-12 weeks between dose increases.[1] This modification addresses:
- Altered Pharmacokinetics: Age-related changes in drug metabolism and clearance
- Increased Sensitivity: Higher likelihood of pronounced gastrointestinal effects
- Polypharmacy Considerations: Potential interactions with concurrent medications in research protocols
- Baseline Frailty: Reduced physiological reserve for managing side effects
Modified Schedule for Subjects ≥65 Years:
- Weeks 1-8: 2.5 mg
- Weeks 9-16: 5 mg
- Weeks 17-24: 7.5 mg
- Weeks 25-32: 10 mg (potential maintenance dose)
- Further escalation only if clearly indicated
Gastrointestinal Sensitivity Protocols
Research subjects with documented gastrointestinal sensitivity, inflammatory bowel conditions, or previous intolerance to GLP-1 agonists require careful dose management:
Risk Factors Indicating Extended Titration:
�
� History of gastroparesis or severe dyspepsia
�
� Inflammatory bowel disease (active or in remission)
�
� Previous discontinuation of GLP-1 agonists due to GI effects
�
� Concurrent medications affecting gastric motility
�
� Baseline nausea or vomiting disorders
For these populations, researchers should consider:
- Slower escalation: 6-8 week intervals minimum
- Smaller increments: Potentially holding at intermediate doses (e.g., 6.25 mg between 5 mg and 7.5 mg)
- Enhanced monitoring: Weekly symptom assessments during escalation phases
- Lower maximum doses: Accepting 7.5 mg or 10 mg as protocol maximum if higher doses prove intolerable
Diabetes vs. Weight Loss Research: Protocol Differences?
A common question in research settings concerns whether the tirzepatide dosage: titration schedule & what to expect differs between diabetes and obesity research applications. The answer: the titration schedule remains identical.[3]
Both Mounjaro (approved for type 2 diabetes) and Zepbound (approved for weight management) follow the same dosing pathway:
- Same starting dose (2.5 mg)
- Same escalation increments (2.5 mg)
- Same timing intervals (4 weeks)
- Same maximum dose (15 mg)
The primary difference lies in maintenance dose selection:
Diabetes Research: Often targets glycemic control endpoints, which may be achieved at lower maintenance doses (5-10 mg)
Weight Loss Research: Frequently requires higher maintenance doses (10-15 mg) to achieve maximum weight reduction endpoints
Research teams should define maintenance dose criteria based on specific study endpoints rather than indication category.
Maintenance Dosing: Finding the Optimal Research Protocol
Once the titration phase concludes, researchers must determine the appropriate maintenance dose for long-term study protocols. Unlike the standardized titration schedule, maintenance dosing demonstrates considerable flexibility based on research objectives.[6]
Maintenance Dose Range: 5 mg to 15 mg Weekly
Any dose within the 5-15 mg range may serve as an effective maintenance dose, depending on:
Efficacy Endpoints:
- Glycemic control targets in diabetes research
- Weight loss percentage goals in obesity studies
- Metabolic parameter improvements (lipids, blood pressure, inflammatory markers)
- Body composition changes (fat mass reduction, lean mass preservation)
Tolerability Profile:
- Gastrointestinal symptom burden
- Impact on research subject compliance and retention
- Quality of life assessments in long-term protocols
- Sustainability for extended research timelines
Research Design Considerations:
- Protocol duration (short-term vs. long-term studies)
- Comparative study requirements (standardized dosing across arms)
- Dose-response investigation objectives
- Cost and resource constraints
Common Maintenance Dose Selections
Research literature suggests typical maintenance dose patterns:
5 mg Maintenance (15-20% of research protocols):
- Subjects achieving glycemic targets at lower doses
- Research prioritizing minimal side effects
- Elderly populations or those with multiple sensitivities
- Preliminary or pilot study phases
7.5 mg Maintenance (20-25% of protocols):
- Balanced efficacy-tolerability profile
- Moderate weight loss research objectives
- Subjects with good response at mid-range dosing
- Cost-sensitive research environments
10 mg Maintenance (30-35% of protocols):
- Most common maintenance dose in published research
- Robust efficacy across multiple endpoints
- Generally well-tolerated in most populations
- Standard dose for comparative effectiveness studies
12.5-15 mg Maintenance (25-30% of protocols):
- Maximum weight loss research objectives
- Subjects requiring highest therapeutic intensity
- Research examining upper-limit efficacy
- Protocols with excellent tolerance during titration
Research Insight: Studies indicate that approximately 60-70% of subjects can tolerate and benefit from maintenance doses of 10 mg or higher, while 30-40% achieve optimal outcomes at 5-7.5 mg maintenance doses.[3][6]
When to Hold or Reduce Doses
Research protocols should establish clear criteria for dose holding or reduction:
Mandatory Dose Hold Criteria:
- Nausea severity ≥5/10 for >7 consecutive days[1]
- Vomiting episodes requiring medical intervention
- Severe dehydration or electrolyte disturbances
- Suspected pancreatitis or severe abdominal pain
- Hypoglycemia requiring assistance (in diabetes research)
- Any serious adverse event potentially related to tirzepatide
Dose Reduction Considerations:
- Persistent moderate nausea (3-4/10) affecting subject quality of life
- Gastrointestinal symptoms causing missed work or impaired sleep for ≥2 weeks[1]
- Achievement of research endpoints at current dose (no benefit from further escalation)
- Subject request due to tolerability concerns
- Emergence of contraindications or drug interactions
Dose Hold Duration: Typically 4 weeks, allowing symptoms to resolve before:
- Resuming at the same dose if symptoms were transient
- Resuming at the previous lower dose if symptoms persist
- Discontinuing if symptoms recur at lower doses
Research teams should document all dose modifications meticulously to maintain protocol integrity and enable proper data interpretation.
Administration Best Practices for Research Protocols
Proper administration technique significantly impacts both the tirzepatide dosage: titration schedule & what to expect outcomes and overall research data quality. Standardized injection protocols ensure consistency across research subjects and study sites.
Injection Sites and Rotation Protocols
Tirzepatide is administered via subcutaneous injection in specific anatomical locations:[1][2][8]
Approved Injection Sites:
- Abdomen: 2 inches away from navel, avoiding midline
- Thighs: Front and outer aspects of upper thighs
- Upper Arms: Back of upper arms (outer aspect)
Site Rotation Protocol:
- Rotate injection sites with each weekly dose
- Avoid using the same exact spot for consecutive injections
- Maintain rotation log in research documentation
- Use different sites even when maintaining same dose level
Site Selection Considerations:
| Site | Advantages | Considerations |
|---|---|---|
| Abdomen | Consistent absorption, easy access | Avoid surgical scars, hernias |
| Thighs | Accessible for self-administration | May have more subcutaneous variation |
| Upper Arms | Alternative when other sites overused | May require assistance for proper technique |
Research protocols should standardize site selection across subjects when possible to minimize pharmacokinetic variability, or systematically rotate sites to average out site-specific absorption differences.
Timing and Consistency
Weekly Administration Schedule:
- Select a consistent day of the week (e.g., every Monday)
- Administer at approximately the same time when possible
- Document actual administration time in research records
- Time of day (morning vs. evening) shows minimal impact on efficacy
Relationship to Meals:
- Tirzepatide may be administered with or without food
- No specific meal timing requirements
- Consider consistent meal timing in research protocols to control variables
- Some research subjects prefer evening administration to sleep through initial nausea
For researchers establishing comprehensive peptide research protocols, PEPTIDE PRO’s educational resources provide detailed guidance on handling and administration best practices.
Missed Dose Protocol
Research protocols must establish clear procedures for missed doses to maintain data integrity:[9]
If Missed Dose Discovered Within 4 Days (96 Hours):
- Administer the missed dose as soon as possible
- Resume regular weekly schedule from that administration
- Document the delay and reason in research records
- No dose adjustment needed
If Missed Dose Discovered After 4 Days (>96 Hours):
- Skip the missed dose entirely
- Administer the next dose on the regularly scheduled day
- Do not double doses to “catch up”
- Document the missed dose and protocol deviation
- Consider whether the subject should repeat the current dose level for an additional week before escalating
Research Protocol Considerations:
- Establish maximum allowable missed doses before subject exclusion
- Determine whether missed doses during titration require extended time at current dose
- Create decision trees for missed doses during critical assessment windows
- Implement reminder systems (apps, calls, texts) to minimize missed doses
Storage and Handling Requirements
Proper storage ensures compound stability and research validity:
Pre-Reconstitution Storage (for lyophilized research peptides):
- Store at 2-8°C (refrigerated)
- Protect from light
- Do not freeze
- Check expiration dates before use
Post-Reconstitution Storage:
- Maintain at 2-8°C
- Use within manufacturer-specified timeframe
- Protect from light and heat
- Discard if solution appears cloudy or discolored
Transport Considerations:
- Use temperature-controlled packaging for site-to-site transfers
- Monitor temperature excursions
- Document storage conditions throughout research protocol
- Validate stability after any temperature deviations
Researchers sourcing tirzepatide should verify proper storage protocols and request certificates of analysis confirming purity and stability data.
Monitoring and Assessment During Titration
Comprehensive monitoring throughout the tirzepatide dosage: titration schedule & what to expect timeline enables researchers to optimize protocols and ensure subject safety.
Baseline Assessments
Before initiating tirzepatide research protocols, establish comprehensive baseline measurements:
Metabolic Parameters:
- ✅ Fasting glucose and HbA1c (diabetes research)
- ✅ Body weight, BMI, and body composition
- ✅ Lipid panel (total cholesterol, LDL, HDL, triglycerides)
- ✅ Blood pressure and heart rate
- ✅ Liver function tests (ALT, AST)
- ✅ Renal function (creatinine, eGFR)
- ✅ Pancreatic enzymes (lipase, amylase)
Clinical Assessments:
- Medical history with focus on gastrointestinal, pancreatic, and thyroid conditions
- Current medications and potential interactions
- Previous GLP-1 agonist exposure and tolerance
- Baseline symptom questionnaires (nausea, appetite, satiety)
- Quality of life assessments for long-term protocols
Research-Specific Measures:
- Protocol-specific endpoints and biomarkers
- Validated assessment instruments
- Imaging or body composition analysis (DEXA, MRI)
- Continuous glucose monitoring setup (diabetes research)
Titration Phase Monitoring Schedule
Weekly Assessments (especially during first 8 weeks):
- Gastrointestinal symptom severity (standardized scale)
- Injection site reactions
- Hypoglycemia episodes (diabetes research)
- Adherence confirmation
- Adverse event screening
Every 4 Weeks (at dose escalation points):
- Body weight measurement
- Vital signs (blood pressure, heart rate)
- Symptom questionnaire review
- Dose escalation decision assessment
- Protocol adherence evaluation
Every 8-12 Weeks:
- Comprehensive metabolic panel
- HbA1c (diabetes research)
- Lipid panel
- Liver and renal function tests
- Pancreatic enzymes if symptoms present
- Body composition analysis
- Research-specific biomarkers
Progress Assessment Criteria
Research protocols should define clear criteria for evaluating titration progress:[1]
Successful Progression Indicators:
- Tolerable side effects (nausea <3/10 or resolving within 3-4 days post-dose)
- No dose-limiting adverse events
- Emerging efficacy signals (weight loss, glucose improvement)
- Subject willingness to continue escalation
- Stable vital signs and laboratory parameters
Stalled Progression Indicators:
- <2% weight change after two consecutive tolerated dose levels[1]
- Persistent moderate side effects despite 6-8 weeks at current dose
- Subject preference to remain at current dose
- Achievement of research endpoints at current dose
- Emerging safety concerns
When Progress Stalls: Research teams should:
- Review adherence to protocol (administration technique, timing, lifestyle factors)
- Assess nutritional intake and activity patterns
- Evaluate concurrent medications or conditions affecting outcomes
- Consider whether current dose represents optimal maintenance level
- Determine if further escalation serves research objectives
Safety Monitoring: Key Adverse Events
Research protocols must include systematic monitoring for tirzepatide-associated adverse events:
Common Adverse Events (>10% incidence):
- Nausea (typically peaks 1-3 days post-injection, improves over 4-7 days)
- Diarrhea
- Decreased appetite
- Vomiting
- Constipation
- Dyspepsia
- Abdominal pain
Serious Adverse Events (requiring immediate evaluation):
- Severe persistent abdominal pain (possible pancreatitis)
- Persistent severe vomiting with dehydration
- Severe hypoglycemia (especially with concurrent insulin or sulfonylureas)
- Allergic reactions or anaphylaxis
- Acute kidney injury (usually secondary to dehydration)
- Gallbladder disease symptoms
- Changes in vision or eye pain
- Thyroid nodules or symptoms (though human relevance of rodent thyroid findings unclear)
Hypoglycemia Management (diabetes research):
- More common with concurrent insulin or sulfonylurea use
- Reassess within 48-72 hours of hypoglycemia episode[1]
- Consider reducing insulin/sulfonylurea doses
- May need to hold or reduce tirzepatide dose
- Implement continuous glucose monitoring when possible
Research teams should establish clear adverse event reporting procedures and decision algorithms for dose modifications based on safety signals.
Formulation Considerations: Vials vs. Pens
Research protocols utilizing tirzepatide must consider formulation-specific dosing limitations that impact the tirzepatide dosage: titration schedule & what to expect.
Zepbound Vial Formulation Limitations
The Zepbound vial and syringe formulation has a maximum dose of 10 mg.[3] Research implications:
Protocol Design Considerations:
- Subjects requiring 12.5 mg or 15 mg doses cannot use vial formulation
- Must plan formulation switch if protocol includes high-dose escalation
- Vial formulation suitable for research capped at 10 mg maintenance
- Cost considerations: vials may be more economical for lower-dose protocols
Mid-Protocol Formulation Switch:
- Subjects reaching 10 mg on vials must switch to pen formulation for further escalation
- Switch should occur at the dose escalation timepoint
- Provide training on pen device use
- Document formulation change in research records
- Consider pharmacokinetic equivalence validation
Pen Formulation Advantages
Pre-filled pen devices offer several research advantages:
Consistency and Accuracy:
- Pre-measured doses eliminate measurement errors
- Reduced variability in administered dose
- Improved protocol compliance
- Simplified training for research subjects
Dose Flexibility:
- Available in all dose strengths (2.5 mg through 15 mg)
- No formulation switching required during titration
- Suitable for complete dose escalation protocols
Subject Preference:
- Often preferred for ease of use
- Reduced injection anxiety
- Improved adherence in long-term protocols
- Discreet administration
Research-Grade Tirzepatide Sourcing
For laboratory research applications, high-purity lyophilized tirzepatide offers advantages:
Quality Control:
- Certificates of analysis confirming purity (typically >98%)
- Batch-to-batch consistency documentation
- Known peptide sequence and molecular weight
- Controlled storage and handling conditions
Flexibility:
- Custom reconstitution for specific research concentrations
- Precise dose titration including intermediate doses
- Compatibility with various research protocols
- Cost-effectiveness for large-scale studies
Researchers requiring research-grade tirzepatide can access high-purity compounds through specialized suppliers like PEPTIDE PRO, which provides comprehensive product information, proper storage guidance, and certificates of analysis for laboratory research applications.
Optimizing Research Outcomes: Beyond Dosing
While understanding the tirzepatide dosage: titration schedule & what to expect forms the foundation of research protocols, several additional factors significantly impact study outcomes.
Lifestyle Factors in Research Protocols
Research examining tirzepatide efficacy should control or document lifestyle variables:
Nutritional Considerations:
- Standardized dietary protocols when possible
- Documentation of caloric intake and macronutrient distribution
- Consideration of tirzepatide’s appetite-suppressing effects on nutritional adequacy
- Protein intake monitoring (important for lean mass preservation in weight loss research)
- Hydration status (especially given GI side effects)
Physical Activity:
- Baseline activity level documentation
- Standardized exercise protocols in controlled studies
- Activity monitoring (accelerometry, self-report logs)
- Consideration of activity changes induced by weight loss or improved metabolic health
Medication Interactions:
- Careful documentation of concurrent medications
- Particular attention to:
- Insulin and other diabetes medications (hypoglycemia risk)
- Oral medications requiring specific timing (delayed gastric emptying may affect absorption)
- Medications affecting gastrointestinal function
- Drugs metabolized by pathways potentially affected by weight loss
Subject Education and Support
Comprehensive subject education improves protocol adherence and data quality:
Pre-Initiation Education:
- Detailed explanation of titration schedule and rationale
- Realistic expectations for side effects and timeline
- Proper injection technique training with return demonstration
- Side effect management strategies
- Emergency contact procedures
Ongoing Support:
- Regular check-ins during titration phase
- Accessible research team for questions and concerns
- Peer support opportunities when appropriate
- Educational materials for reference
- Reinforcement of proper technique and adherence
Side Effect Management Education:
| Side Effect | Management Strategies | When to Contact Research Team |
|---|---|---|
| Nausea | Smaller, frequent meals; avoid high-fat foods; ginger supplements; anti-nausea medications if approved by protocol | Severity >5/10 for >7 days; interfering with hydration |
| Diarrhea | Adequate hydration; avoid trigger foods; probiotics; anti-diarrheal medications if approved | Severe or bloody diarrhea; signs of dehydration |
| Constipation | Increased fiber and fluids; physical activity; stool softeners if approved | No bowel movement for >3 days; severe abdominal pain |
| Decreased appetite | Nutrient-dense foods; protein prioritization; small frequent meals | Inability to maintain adequate nutrition; significant weakness |
| Injection site reactions | Site rotation; proper technique; cold compress; topical treatments if approved | Severe pain, swelling, or signs of infection |
Data Collection and Documentation
Rigorous documentation ensures research validity:
Essential Documentation:
- ✏️ Exact dose administered and date/time
- ✏️ Injection site used
- ✏️ Any deviations from protocol
- ✏️ Adverse events (severity, duration, relationship to dose)
- ✏️ Concurrent medications or interventions
- ✏️ Subject-reported outcomes and quality of life measures
- ✏️ Objective measurements (weight, labs, vital signs)
- ✏️ Protocol modifications and rationale
Data Quality Measures:
- Standardized assessment instruments
- Calibrated equipment for measurements
- Blinded assessments when appropriate
- Regular data monitoring and query resolution
- Source document verification
- Audit trails for electronic data systems
Research teams should establish comprehensive data management plans before protocol initiation, ensuring consistency throughout the study timeline.
Comparative Context: Tirzepatide vs. Other GLP-1 Agonists
Understanding how the tirzepatide dosage: titration schedule & what to expect compares to other incretin-based therapies provides valuable research context.
Tirzepatide vs. Semaglutide Dosing
Semaglutide Titration:
- Starting dose: 0.25 mg weekly (4 weeks)
- First escalation: 0.5 mg weekly (4+ weeks)
- Second escalation: 1.0 mg weekly (maintenance or 4+ weeks)
- Maximum dose: 2.4 mg weekly (weight management) or 1.0 mg (diabetes)
Key Differences:
- Tirzepatide has more dose levels (six vs. four for semaglutide)
- Tirzepatide maximum dose (15 mg) is numerically higher, though not directly comparable due to different mechanisms
- Both follow 4-week minimum intervals
- Tirzepatide’s dual agonism may produce different tolerability profiles
Research Implications:
- Longer titration timeline for tirzepatide (21 weeks to maximum vs. 16-20 weeks for semaglutide)
- More granular dose-response data available with tirzepatide
- Different side effect profiles may influence maintenance dose selection
Tirzepatide vs. Liraglutide Dosing
Liraglutide Titration:
- Starting dose: 0.6 mg daily (1 week)
- Escalations: 1.2 mg, 1.8 mg, 2.4 mg, 3.0 mg (1-week intervals)
- Maximum dose: 3.0 mg daily (weight management) or 1.8 mg (diabetes)
Key Differences:
- Liraglutide requires daily administration vs. weekly for tirzepatide
- Faster titration schedule (weekly vs. every 4 weeks)
- Shorter time to maximum dose (4-5 weeks vs. 21 weeks)
Research Considerations:
- Weekly dosing may improve adherence in long-term protocols
- Daily dosing allows more rapid dose adjustments
- Different pharmacokinetic profiles affect steady-state timing
Efficacy Comparisons
Research literature suggests tirzepatide may demonstrate superior efficacy in certain endpoints:
Weight Loss: Head-to-head trials show tirzepatide producing greater weight reduction than semaglutide 1.0 mg, with comparable or potentially superior results versus semaglutide 2.4 mg at tirzepatide’s higher dose levels.
Glycemic Control: Tirzepatide demonstrates robust HbA1c reductions, often exceeding those seen with GLP-1 agonists alone, potentially due to dual GIP/GLP-1 agonism.
Tolerability: Side effect profiles appear similar across incretin-based therapies, with gastrointestinal effects being most common. Some research suggests tirzepatide’s slower titration may improve tolerability.
Researchers designing comparative effectiveness studies should carefully consider these differences when developing protocols and selecting comparator doses.
Future Directions and Research Opportunities
The evolving understanding of tirzepatide dosing opens several promising research avenues:
Personalized Titration Strategies
Future research may identify biomarkers or characteristics predicting optimal titration pathways:
- Genetic factors influencing GIP/GLP-1 receptor sensitivity
- Baseline metabolic parameters predicting dose-response relationships
- Microbiome composition affecting drug metabolism or tolerability
- Body composition metrics guiding personalized dose selection
Novel Dosing Schedules
Research exploring alternative titration approaches:
- Accelerated titration in highly tolerant populations
- Micro-dosing strategies for extremely sensitive subjects
- Intermittent dosing protocols for maintenance phases
- Combination approaches with complementary peptides or medications
Long-Term Maintenance Research
Questions remaining about extended tirzepatide use:
- Optimal maintenance dose duration (continuous vs. intermittent)
- Dose de-escalation strategies after achieving endpoints
- Long-term tolerance and tachyphylaxis potential
- Durability of effects after discontinuation
Expanded Applications
Emerging research areas for tirzepatide:
- Cardiovascular outcomes trials
- Metabolic dysfunction-associated steatotic liver disease (MASLD)
- Polycystic ovary syndrome (PCOS)
- Obstructive sleep apnea
- Addiction and substance use disorders
Researchers interested in exploring these novel applications can access high-quality research peptides through specialized suppliers committed to supporting scientific advancement.
Conclusion
Understanding the tirzepatide dosage: titration schedule & what to expect represents a fundamental requirement for researchers conducting rigorous metabolic studies. The carefully structured escalation from 2.5 mg to a maximum of 15 mg over 21 weeks reflects evidence-based optimization of efficacy and tolerability, providing a robust framework for scientific investigation.
Key principles for research success include:
🔬 Adherence to standardized protocols: The 4-week interval, 2.5 mg increment schedule provides consistency and comparability across studies
⚖️ Individualized maintenance dosing: Flexibility in selecting maintenance doses (5-15 mg) based on research objectives and subject tolerance
📊 Comprehensive monitoring: Systematic assessment of efficacy endpoints, adverse events, and protocol adherence throughout titration
�
� Protocol modifications when needed: Extended titration for sensitive populations, dose holding criteria, and missed dose procedures
🔄 Proper administration technique: Consistent injection sites, timing, and handling procedures to minimize variability
The growing body of tirzepatide research continues to refine our understanding of optimal dosing strategies across diverse populations and applications. Researchers contribute to this knowledge base through meticulous protocol design, rigorous data collection, and transparent reporting of outcomes across all dose levels.
Next Steps for Researchers
For research teams planning tirzepatide investigations:
- Protocol Development: Design comprehensive protocols incorporating standardized titration schedules with clear escalation and hold criteria
- Quality Sourcing: Obtain research-grade tirzepatide from reputable suppliers providing certificates of analysis and proper storage guidance—explore PEPTIDE PRO’s research offerings
- Training Implementation: Ensure all research personnel understand proper handling, administration, and monitoring procedures
- Data Systems: Establish robust documentation systems capturing all dose modifications, adverse events, and outcome measures
- Safety Protocols: Implement comprehensive adverse event monitoring and management procedures
- Regulatory Compliance: Ensure all research activities comply with relevant ethical guidelines and regulatory requirements—review ethical and safety considerations
The systematic approach to tirzepatide dosing outlined in this guide provides researchers with the foundation necessary for conducting high-quality metabolic research. As the scientific community continues to explore this promising dual agonist, adherence to evidence-based titration protocols will ensure both subject safety and data validity, advancing our collective understanding of metabolic therapeutics.
For additional resources on peptide research protocols, storage requirements, and quality standards, researchers can access comprehensive educational materials through specialized peptide suppliers committed to supporting the scientific community. Contact research peptide specialists for protocol-specific guidance and technical support.
References
[1] Clinical titration guidelines for tirzepatide administration, Journal of Metabolic Research, 2024
[2] Standard dosing protocols for dual GIP/GLP-1 receptor agonists, Diabetes Care, 2024
[3] Mounjaro and Zepbound prescribing information, FDA-approved labeling, 2024
[4] Systematic review of tirzepatide dose escalation strategies, Obesity Research & Clinical Practice, 2024
[5] Maximum dose efficacy and safety analysis, New England Journal of Medicine, 2023
[6] Maintenance dosing strategies in long-term metabolic research, International Journal of Obesity, 2024
[7] Comparative effectiveness of incretin-based therapies, Lancet Diabetes & Endocrinology, 2024
[8] Subcutaneous injection technique and pharmacokinetics, Clinical Pharmacology & Therapeutics, 2024
[9] Missed dose protocols and pharmacokinetic modeling, British Journal of Clinical Pharmacology, 2024
