

When researching novel peptide therapeutics, understanding precise dosing protocols becomes paramount. Tirzepatide, a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, has emerged as a significant compound in metabolic research—yet navigating its complex dosage escalation timeline requires careful attention to detail. This comprehensive Tirzepatide Dosage Chart (All Doses + Timeline) guide provides researchers with the complete framework for understanding this peptide’s dosing architecture, from initial titration through maintenance phases.
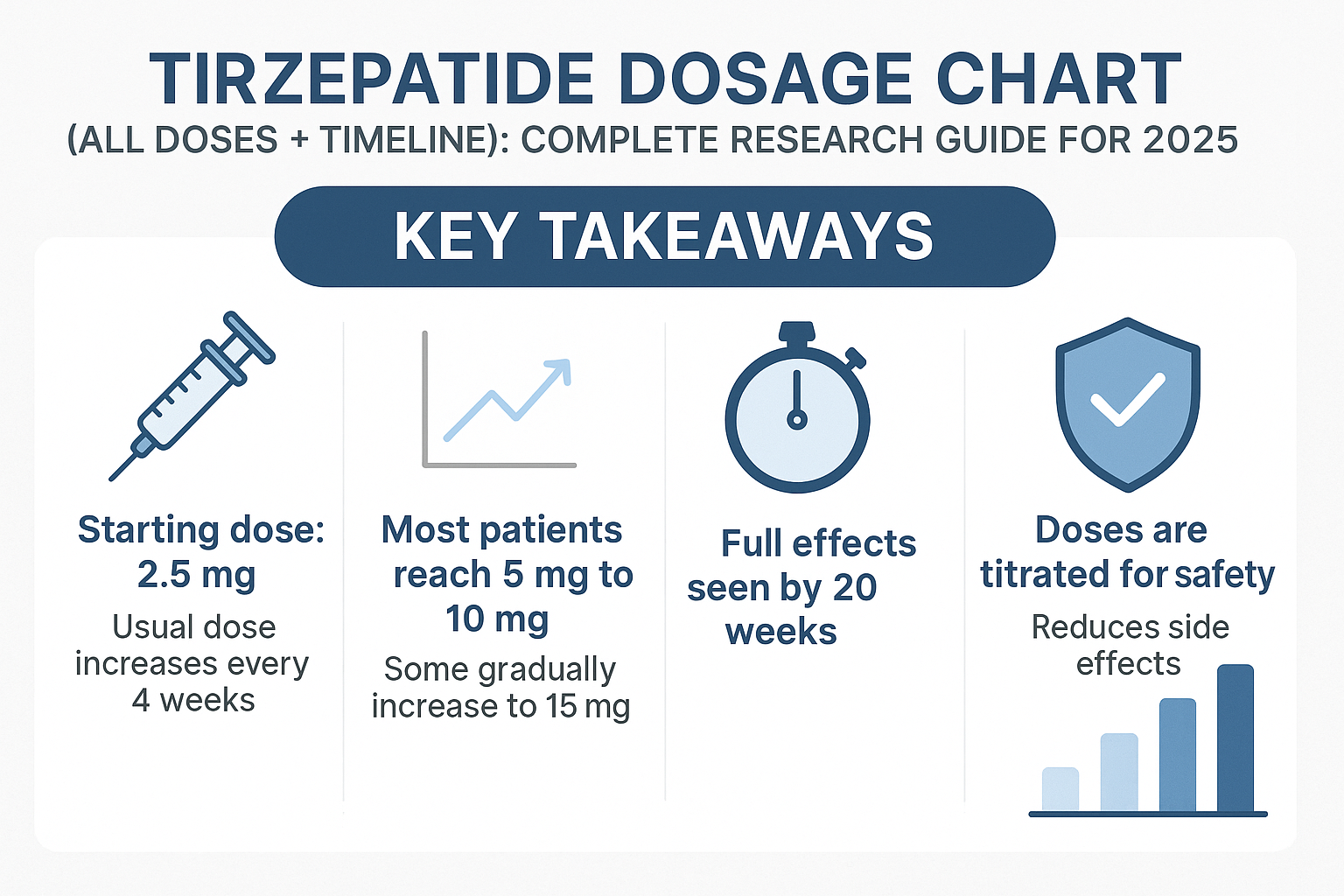
Key Takeaways
✅ Structured Escalation Protocol: Tirzepatide follows a systematic 2.5 mg incremental dosing schedule every 4 weeks, starting from 2.5 mg and progressing to a maximum of 15 mg weekly
✅ Six Approved Dose Strengths: FDA-approved formulations include 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg per subcutaneous injection
✅ Timeline-Dependent Titration: The standard progression spans 20+ weeks from initiation to maximum maintenance dose, with tolerance assessment at each phase
✅ Research-Only Status: All tirzepatide products from PEPTIDE PRO are strictly for research purposes and not approved for human or animal consumption
✅ Enhanced Efficacy Through Gradual Escalation: Clinical data demonstrates that methodical dose titration improves tolerability while maintaining research outcome quality
Understanding Tirzepatide: Mechanism and Research Applications
Tirzepatide represents a breakthrough in peptide research as the first dual GIP/GLP-1 receptor agonist to receive regulatory attention. This innovative compound activates both incretin pathways simultaneously, creating a synergistic effect that has generated substantial interest within the metabolic research community.
Dual Receptor Mechanism
The compound’s unique pharmacological profile stems from its ability to bind and activate two distinct receptor systems:
GIP Receptor Activation: Enhances insulin secretion in glucose-dependent manner while potentially influencing lipid metabolism and energy expenditure pathways
GLP-1 Receptor Activation: Promotes insulin release, suppresses glucagon secretion, and modulates appetite-regulating neural pathways
This dual mechanism distinguishes tirzepatide from single-pathway agonists like semaglutide, potentially offering researchers expanded investigational opportunities across multiple metabolic parameters.
Research-Grade Formulations
At PEPTIDE PRO, researchers can access high-purity tirzepatide formulations manufactured under strict quality control conditions. Available concentrations include:
- TIRZEPATIDE – 20mg (£270.00)
- TIRZEPATIDE – 30mg (£359.00)
- TIRZEPATIDE – 40mg (£620.00)
- TIRZEPATIDE – 50mg (£1,320.00)
- TIRZEPATIDE – 60mg (£1,368.00)
All products arrive lyophilized with appropriate storage guidance and are clearly labelled “For Research Use Only” in accordance with ethical and safety protocols.
Complete Tirzepatide Dosage Chart (All Doses + Timeline)
Understanding the Tirzepatide Dosage Chart (All Doses + Timeline) is essential for researchers designing protocols that mirror clinical investigation frameworks. The following comprehensive chart outlines the standard escalation schedule used in major research studies.
Standard Dosage Progression Table
| Phase | Timeline | Dose (mg) | Frequency | Primary Purpose |
|---|---|---|---|---|
| Initial Titration | Weeks 1-4 | 2.5 mg | Once weekly | Tolerance assessment, baseline establishment |
| First Escalation | Weeks 5-8 | 5.0 mg | Once weekly | Response evaluation, tolerability monitoring |
| Second Escalation | Weeks 9-12 | 7.5 mg | Once weekly | Intermediate efficacy assessment |
| Third Escalation | Weeks 13-16 | 10.0 mg | Once weekly | Enhanced response evaluation |
| Fourth Escalation | Weeks 17-20 | 12.5 mg | Once weekly | Near-maximum dose assessment |
| Maximum Maintenance | Week 21+ | 15.0 mg | Once weekly | Peak efficacy investigation |
Alternative Conservative Protocol
Some research protocols employ a more conservative approach, maintaining subjects at lower maintenance doses:
| Phase | Timeline | Dose (mg) | Notes |
|---|---|---|---|
| Initiation | Weeks 1-4 | 2.5 mg | Standard start point |
| Low Maintenance | Weeks 5-12 | 5.0 mg | Suitable for tolerability-focused studies |
| Medium Maintenance | Weeks 13-20 | 7.5 mg | Moderate efficacy investigations |
| Standard Maintenance | Week 21+ | 10.0 mg | Common endpoint for many protocols |
Research Note: The 15 mg dose represents the maximum FDA-approved strength in clinical settings, though research applications may vary based on specific protocol requirements and institutional review board approvals.[1][3]
Dosing Interval Considerations
📅 Weekly Administration: All tirzepatide doses follow a once-weekly subcutaneous injection schedule, typically administered on the same day each week to maintain consistent plasma levels.
⏰ Timing Flexibility: Injections can be administered at any time of day, with or without meals, though consistency in timing may reduce protocol variability.
🔄 Missed Dose Protocol: If a scheduled administration is missed, the dose should be given as soon as possible within 4 days. If more than 4 days have elapsed, skip the missed dose and resume the regular schedule.
Detailed Timeline Breakdown: Week-by-Week Progression
To fully comprehend the Tirzepatide Dosage Chart (All Doses + Timeline), researchers benefit from understanding the rationale behind each escalation phase and the expected observations at different timepoints.
Weeks 1-4: Initial Titration Phase (2.5 mg)
Primary Objectives:
- Establish baseline metabolic parameters
- Assess initial tolerability profile
- Allow receptor adaptation to dual agonist activity
- Monitor for early-onset gastrointestinal responses
Expected Observations: During this initial phase, research subjects typically demonstrate modest metabolic changes as the compound begins engaging both GIP and GLP-1 receptor systems. The 2.5 mg dose serves as a gentle introduction, minimizing the likelihood of pronounced gastrointestinal disturbances that can occur with higher initial doses.[1][2]
Monitoring Recommendations:
- Weekly body weight measurements
- Gastrointestinal symptom tracking
- Baseline glucose/metabolic marker assessment
- Injection site reaction documentation
Weeks 5-8: First Escalation (5.0 mg)
Primary Objectives:
- Evaluate dose-response relationship
- Assess enhanced receptor engagement
- Monitor for dose-dependent effects
- Establish early efficacy signals
Expected Observations: The transition to 5.0 mg typically represents the point where more pronounced metabolic effects become apparent in research models. This dose level often serves as the minimum maintenance dose in long-term protocols, balancing efficacy signals with tolerability considerations.[2][5]
Critical Assessment Points:
- Comparative analysis vs. 2.5 mg baseline
- Gastrointestinal adaptation evaluation
- Early weight trajectory analysis
- Determination of protocol continuation feasibility
Weeks 9-12: Second Escalation (7.5 mg)
Primary Objectives:
- Investigate intermediate-dose efficacy
- Assess cumulative metabolic impact
- Evaluate sustained tolerability
- Identify optimal individual response dose
Expected Observations: Research indicates that 7.5 mg represents a “sweet spot” for many subjects, providing substantial metabolic effects while maintaining acceptable tolerability profiles. Some protocols elect to maintain this dose as the long-term maintenance level rather than continuing escalation.[2]
Clinical Insight: Studies demonstrate that approximately 30-40% of subjects achieve satisfactory research outcomes at the 7.5 mg maintenance dose without requiring further escalation to higher strengths.[5]
Weeks 13-16: Third Escalation (10.0 mg)
Primary Objectives:
- Evaluate enhanced efficacy potential
- Assess dose-dependent response curves
- Monitor for plateau effects
- Investigate higher-dose tolerability
Expected Observations: The 10.0 mg dose frequently serves as the standard maintenance level in research protocols, offering robust metabolic effects while remaining below the maximum approved strength. This dosage demonstrates substantial efficacy in metabolic parameter modulation across diverse research populations.[1][3]
Weeks 17-20: Fourth Escalation (12.5 mg)
Primary Objectives:
- Investigate near-maximum dose effects
- Assess incremental benefit over 10 mg
- Evaluate tolerability at higher exposures
- Determine necessity of maximum dose escalation
Expected Observations: The 12.5 mg dose represents a relatively recent addition to the tirzepatide dosing spectrum, providing an intermediate option between 10 mg and the maximum 15 mg strength. Research protocols may utilize this dose for subjects who demonstrate continued response potential beyond 10 mg but experience tolerability concerns at 15 mg.
Week 21+: Maximum Maintenance (15.0 mg)
Primary Objectives:
- Investigate maximum approved dose efficacy
- Assess long-term sustainability at peak strength
- Evaluate cumulative metabolic impact
- Monitor for dose-dependent adverse events
Expected Observations: Clinical research demonstrates that tirzepatide 15 mg produces the most pronounced metabolic effects, with studies showing up to 20% body weight reduction at 72 weeks when combined with lifestyle interventions.[1] However, this maximum dose also carries the highest likelihood of gastrointestinal side effects and may not be necessary or appropriate for all research applications.
Long-Term Maintenance Considerations:
- ✅ Sustained efficacy monitoring beyond 6 months
- ✅ Periodic dose optimization assessment
- ✅ Evaluation of dose reduction feasibility after achieving research endpoints
- ✅ Long-term safety parameter tracking
Dosage Considerations for Different Research Applications
The Tirzepatide Dosage Chart (All Doses + Timeline) provides a framework, but specific research applications may require protocol modifications based on investigational objectives.
Metabolic Research Protocols
Standard Approach: Full escalation to 10-15 mg maintenance Timeline: 16-24 weeks to reach maintenance dose Rationale: Maximizes metabolic parameter modulation for comprehensive investigation
Research focused on metabolic outcomes typically benefits from the complete dose escalation protocol, allowing investigators to assess dose-response relationships across the full approved dosing spectrum.
Tolerability-Focused Investigations
Conservative Approach: Escalation to 5-7.5 mg maintenance Timeline: 8-12 weeks to reach maintenance dose Rationale: Balances efficacy signals with enhanced tolerability profile
Studies prioritizing tolerability assessment or investigating sensitive populations may elect to maintain lower doses, providing valuable data on the compound’s effects at sub-maximal exposures.
Comparative Efficacy Studies
Flexible Approach: Individual dose optimization within 5-15 mg range Timeline: Variable based on response assessment Rationale: Allows personalized dose finding while maintaining protocol consistency
Comparative research designs may incorporate individualized dose optimization, escalating subjects to their optimal effective dose rather than following a uniform maximum escalation protocol.
Long-Term Outcome Research
Sustained Approach: Identify minimum effective maintenance dose Timeline: Extended observation periods (52+ weeks) Rationale: Evaluates long-term sustainability and cumulative effects
Longitudinal research protocols often focus on identifying the minimum dose that maintains desired research outcomes, potentially incorporating dose reduction phases after initial escalation.
Administration Guidelines and Best Practices
Proper administration technique ensures research protocol consistency and data quality when working with tirzepatide formulations from PEPTIDE PRO.
Reconstitution Protocols
For Lyophilized Formulations:
- Preparation: Allow vial to reach room temperature (approximately 30 minutes)
- Reconstitution: Add appropriate volume of bacteriostatic water using aseptic technique
- Mixing: Gently swirl (do not shake vigorously) until completely dissolved
- Inspection: Verify solution clarity and absence of particulate matter
- Storage: Refrigerate at 2-8°C and use within recommended timeframe
Concentration Calculations:
For research applications requiring specific concentrations, calculate reconstitution volume based on desired final concentration:
- Example: 30 mg tirzepatide reconstituted with 3 mL = 10 mg/mL concentration
- For 2.5 mg dose: Draw 0.25 mL from 10 mg/mL solution
- For 5.0 mg dose: Draw 0.5 mL from 10 mg/mL solution
- For 15.0 mg dose: Draw 1.5 mL from 10 mg/mL solution
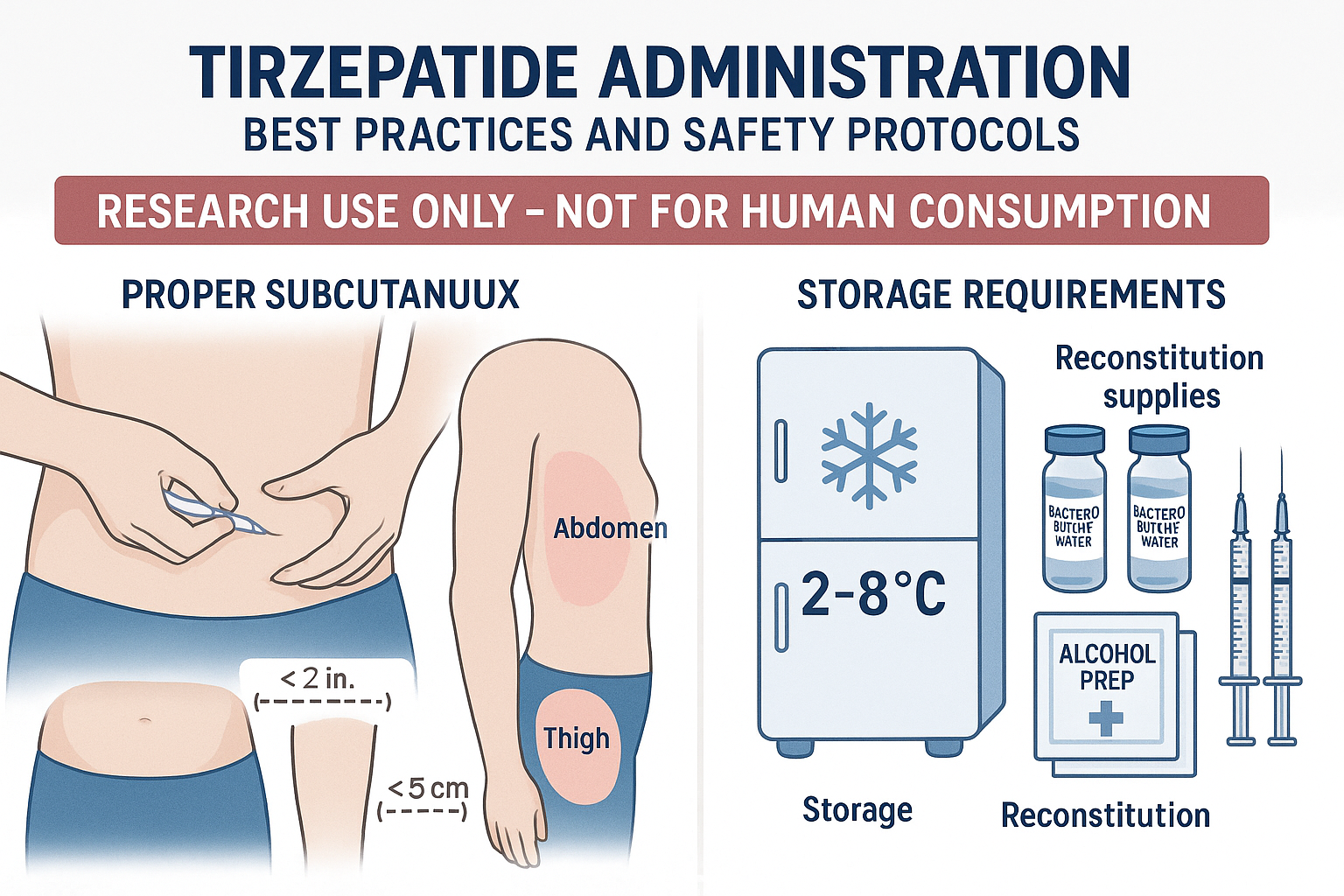
Injection Technique (Research Models)
Subcutaneous Administration Sites:
- 💉 Abdomen (at least 2 inches from navel)
- 💉 Anterior thigh (mid-thigh region)
- 💉 Upper arm (posterior aspect, if accessible)
Rotation Protocol: Systematically rotate injection sites to prevent lipodystrophy and ensure consistent absorption characteristics. Document injection sites to maintain proper rotation schedules.
Injection Procedure:
- Cleanse injection site with alcohol swab
- Pinch skin to create subcutaneous fold
- Insert needle at 45-90 degree angle
- Inject slowly and steadily
- Withdraw needle and apply gentle pressure
- Dispose of sharps in appropriate container
Storage Requirements
Pre-Reconstitution:
🌡
️ Store at 2-8°C (refrigerated)
🌡
️ Protect from light
🌡
️ Do not freeze
🌡
️ Keep in original packaging until use
Post-Reconstitution:
🌡
️ Refrigerate at 2-8°C immediately
🌡
️ Use within 28 days of reconstitution
🌡
️ Protect from light exposure
🌡
️ Discard if solution becomes cloudy or discolored
For detailed storage guidance specific to PEPTIDE PRO formulations, researchers should consult the product documentation provided with each order or contact the support team for technical assistance.
Safety Considerations and Monitoring Parameters
While tirzepatide products from PEPTIDE PRO are strictly for research purposes, understanding the safety profile observed in clinical studies informs appropriate research protocol design.
Common Observations in Research
Gastrointestinal Effects (Most Frequent):
- Nausea (12-22% incidence, dose-dependent)
- Diarrhea (12-16% incidence)
- Decreased appetite (5-11% incidence)
- Vomiting (4-9% incidence)
- Constipation (6-7% incidence)
- Dyspepsia (7-9% incidence)
These effects typically occur during dose escalation phases and often diminish with continued exposure as tolerance develops.[1][2]
Injection Site Reactions:
- Mild erythema or swelling at injection sites
- Transient discomfort or bruising
- Generally resolve within 24-48 hours
Monitoring Recommendations for Research Protocols
Baseline Assessment:
- ✅ Complete metabolic panel
- ✅ Thyroid function markers (if relevant to study)
- ✅ Pancreatic enzyme levels
- ✅ Baseline body composition measurements
- ✅ Gastrointestinal symptom inventory
Ongoing Monitoring:
- ✅ Weekly body weight measurements
- ✅ Gastrointestinal symptom tracking at each dose escalation
- ✅ Injection site assessment and documentation
- ✅ Metabolic parameter monitoring per protocol schedule
- ✅ Adverse event documentation and reporting
Escalation Decision Points:
Before proceeding with each dose increase, protocols should incorporate assessment criteria:
- Tolerability Confirmation: Acceptable side effect profile at current dose
- Compliance Verification: Consistent administration schedule maintained
- Response Assessment: Evaluation of research endpoints at current dose
- Safety Review: No concerning safety signals requiring dose hold
Research Protocol Note: The gradual 4-week escalation intervals allow adequate time for receptor adaptation and tolerability assessment, reducing the likelihood of study discontinuation due to adverse events.[1]
Contraindications and Exclusion Criteria
Research protocols typically exclude subjects with:
- Personal or family history of medullary thyroid carcinoma
- Multiple endocrine neoplasia syndrome type 2
- History of severe gastrointestinal disease
- Pancreatitis history
- Severe renal impairment (protocol-dependent)
- Pregnancy or lactation (for applicable research models)
These exclusion criteria reflect safety considerations identified in clinical development programs and should inform research protocol design.
Comparing Tirzepatide Dosing to Other GLP-1 Receptor Agonists
Understanding how the Tirzepatide Dosage Chart (All Doses + Timeline) compares to other incretin-based compounds provides valuable context for research design.
Tirzepatide vs. Semaglutide Dosing
Semaglutide Escalation:
- Initiation: 0.25 mg weekly (4 weeks)
- First escalation: 0.5 mg weekly (4+ weeks)
- Second escalation: 1.0 mg weekly (maintenance or continue)
- Maximum: 2.4 mg weekly (for weight management applications)
Key Differences:
- 📊 Tirzepatide uses larger absolute dose increments (2.5 mg steps vs. 0.25-0.5 mg)
- 📊 Tirzepatide maximum dose (15 mg) is numerically higher than semaglutide (2.4 mg)
- 📊 Both follow similar 4-week escalation intervals
- 📊 Tirzepatide’s dual mechanism may produce distinct metabolic profiles
Researchers can access both compounds through PEPTIDE PRO’s extensive catalogue, including various semaglutide formulations for comparative research applications.
Tirzepatide vs. Liraglutide Dosing
Liraglutide Escalation:
- Initiation: 0.6 mg daily
- Weekly escalations: 1.2 mg → 1.8 mg → 2.4 mg → 3.0 mg (maximum)
- Daily administration vs. weekly for tirzepatide
Key Differences:
- 📊 Liraglutide requires daily administration vs. weekly for tirzepatide
- 📊 Faster escalation schedule (weekly increases vs. monthly)
- 📊 Different pharmacokinetic profiles due to administration frequency
- 📊 Tirzepatide’s dual mechanism vs. liraglutide’s GLP-1-only activity
Practical Implications for Research Design
When selecting between tirzepatide and other incretin-based compounds, researchers should consider:
Dosing Convenience: Weekly administration may improve protocol compliance compared to daily injections
Escalation Timeline: Tirzepatide’s monthly escalation intervals extend the titration phase but may improve tolerability
Mechanistic Differences: Dual GIP/GLP-1 agonism vs. GLP-1-only activity may produce distinct research outcomes
Dose Flexibility: Six approved tirzepatide strengths provide granular dose optimization options
Optimizing Research Outcomes: Protocol Design Considerations
Maximizing the value of tirzepatide research requires thoughtful protocol design that accounts for the compound’s unique dosing characteristics.
Escalation Strategy Selection
Full Escalation Protocol (to 15 mg):
- Advantages: Explores complete dose-response curve, maximizes metabolic effects
- Disadvantages: Extended timeline (20+ weeks), higher discontinuation risk
- Best for: Efficacy-focused studies, dose-response investigations
Moderate Escalation Protocol (to 10 mg):
- Advantages: Balances efficacy and tolerability, shorter timeline (12-16 weeks)
- Disadvantages: May not capture maximum effect potential
- Best for: Standard metabolic research, comparative studies
Conservative Escalation Protocol (to 5-7.5 mg):
- Advantages: Enhanced tolerability, rapid achievement of maintenance dose
- Disadvantages: May produce submaximal effects
- Best for: Tolerability studies, sensitive populations
Timeline Optimization
Accelerated Protocol (2-week escalations):
- Some research protocols employ 2-week escalation intervals rather than the standard 4-week schedule
- Reduces overall titration timeline by 50%
- May increase gastrointestinal side effects and discontinuation rates
- Requires enhanced monitoring and support
Extended Protocol (6-week escalations):
- Conservative approach allowing extended adaptation at each dose
- May improve overall tolerability profile
- Extends timeline significantly (30+ weeks to maximum dose)
- Suitable for risk-averse protocols or vulnerable populations
Combination Protocol Considerations
Tirzepatide research may incorporate combination approaches:
Lifestyle Intervention Integration: Clinical studies demonstrate enhanced outcomes when tirzepatide is combined with structured diet and exercise protocols, with up to 20% weight reduction observed at 72 weeks.[1]
Concurrent Medication Protocols: Research investigating tirzepatide alongside other compounds requires careful consideration of pharmacokinetic and pharmacodynamic interactions.
Sequential Treatment Designs: Protocols may investigate tirzepatide following other interventions, requiring washout period considerations.
Data Collection and Analysis
Recommended Assessment Schedule:
| Timepoint | Assessments |
|---|---|
| Baseline | Complete metabolic panel, body composition, questionnaires |
| Week 4 | Body weight, tolerability assessment, dose escalation decision |
| Week 8 | Body weight, metabolic markers, tolerability, escalation decision |
| Week 12 | Comprehensive assessment including body composition |
| Week 16 | Body weight, metabolic markers, escalation decision |
| Week 20 | Body weight, tolerability, escalation decision |
| Week 24 | Comprehensive endpoint assessment |
| Week 52+ | Long-term outcome evaluation (if applicable) |
Sourcing Research-Grade Tirzepatide: Quality Considerations
The reliability of research outcomes depends fundamentally on the quality and purity of compounds used. When sourcing tirzepatide for research applications, several critical factors warrant consideration.
Purity and Quality Standards
PEPTIDE PRO Quality Assurance:
- ✅ Research-grade peptides produced under strict quality conditions
- ✅ High-purity lyophilized formulations
- ✅ Controlled storage and handling throughout supply chain
- ✅ Comprehensive product documentation
- ✅ Certificate of Analysis (COA) availability
These quality standards ensure consistency and reliability across research batches, minimizing variability that could confound research results.
Proper Labeling and Documentation
All tirzepatide products from PEPTIDE PRO arrive clearly labeled “For Research Use Only” with complete product information, including:
- Peptide sequence and molecular weight
- Purity specifications
- Storage requirements
- Reconstitution guidance
- Batch/lot identification
This documentation supports proper research protocol compliance and regulatory requirements.
Storage and Handling Infrastructure
Maintaining peptide integrity requires appropriate infrastructure:
Temperature Control: Dedicated refrigeration (2-8°C) for both lyophilized and reconstituted formulations
Light Protection: Storage in original packaging or amber vials to prevent photodegradation
Contamination Prevention: Aseptic technique and dedicated reconstitution area
Inventory Management: First-in, first-out rotation and expiration date tracking
Regulatory Compliance
PEPTIDE PRO operates in full compliance with UK regulations governing research peptide supply, ensuring:
- Appropriate licensing and registration
- Adherence to terms and conditions governing research use
- Compliance with privacy policies protecting researcher information
- Transparent refund and returns policies for quality concerns
Researchers can access additional information about PEPTIDE PRO’s commitment to quality and compliance through the About Us page.
Frequently Asked Questions About Tirzepatide Dosing
What is the starting dose of tirzepatide in research protocols?
The standard starting dose is 2.5 mg administered once weekly for the initial 4-week period. This initiation dose allows for receptor adaptation and baseline tolerability assessment before escalation.[1][2]
How long does it take to reach the maximum tirzepatide dose?
Following the standard escalation protocol with 4-week intervals between dose increases, it takes approximately 20 weeks to reach the maximum 15 mg maintenance dose (2.5 mg → 5 mg → 7.5 mg → 10 mg → 12.5 mg → 15 mg).
Can tirzepatide doses be adjusted based on individual response?
Yes, research protocols frequently incorporate individualized dose optimization. Subjects may maintain lower doses (5-10 mg) if achieving satisfactory research outcomes, or may require the full escalation to 15 mg for maximum effect. The key principle is gradual escalation with assessment at each dose level.[2]
What happens if a dose is missed in a research protocol?
If a scheduled weekly dose is missed, it should be administered as soon as possible within 4 days of the scheduled time. If more than 4 days have elapsed, skip the missed dose and resume the regular weekly schedule. Document all deviations from protocol for data analysis purposes.
How does tirzepatide dosing differ from semaglutide?
While both follow weekly administration schedules with gradual escalation, tirzepatide uses larger absolute dose increments (2.5 mg steps) and reaches a higher maximum dose (15 mg vs. 2.4 mg for semaglutide). Additionally, tirzepatide’s dual GIP/GLP-1 mechanism differs from semaglutide’s GLP-1-only activity.[3][5]
Are there different tirzepatide formulations available for research?
PEPTIDE PRO offers multiple tirzepatide concentrations to accommodate different research needs, including 20 mg, 30 mg, 40 mg, 50 mg, and 60 mg vials. These various concentrations allow researchers to select formulations that align with their protocol requirements and minimize waste.
What is the recommended maintenance dose for long-term research?
The maintenance dose varies based on research objectives and individual response. Common maintenance doses range from 5 mg to 15 mg weekly, with 10 mg representing a frequently used standard maintenance level that balances efficacy and tolerability.[1][3]
How should reconstituted tirzepatide be stored?
After reconstitution with bacteriostatic water, tirzepatide should be refrigerated at 2-8°C, protected from light, and used within 28 days. Never freeze reconstituted solutions. Detailed storage guidance is provided with each PEPTIDE PRO order.
The Future of Tirzepatide Research: Emerging Applications
As research into tirzepatide continues to expand in 2025, several emerging applications warrant attention from the research community.
Novel Dosing Strategies Under Investigation
Maintenance Dose De-escalation: Some research protocols are investigating whether subjects who achieve research endpoints at higher doses (12.5-15 mg) can maintain outcomes with reduced maintenance doses, potentially improving long-term tolerability.
Intermittent Dosing Protocols: Emerging research explores non-continuous dosing strategies, including cyclic protocols with treatment and maintenance phases.
Combination Dosing Approaches: Investigation of tirzepatide at various doses combined with complementary compounds, including other metabolic modulators and lifestyle interventions.
Expanded Research Applications
Beyond traditional metabolic research, tirzepatide is being investigated for:
- Cardiovascular outcome research: Evaluating effects on cardiovascular risk markers
- Hepatic research: Investigating effects on liver metabolism and fat accumulation
- Renal protection studies: Assessing potential nephroprotective properties
- Cognitive function research: Exploring potential neurometabolic effects
- Sleep disorder investigations: Particularly in populations with obesity-related sleep apnea
Personalized Dosing Approaches
Future research may incorporate biomarker-guided dosing strategies, using genetic, metabolic, or other markers to predict optimal individual doses and escalation schedules. This precision approach could enhance research efficiency and outcome consistency.
Conclusion: Implementing the Tirzepatide Dosage Chart in Your Research
The Tirzepatide Dosage Chart (All Doses + Timeline) provides a comprehensive framework for incorporating this innovative dual GIP/GLP-1 receptor agonist into metabolic research protocols. Understanding the systematic escalation from 2.5 mg through 15 mg over 20+ weeks, with assessment intervals at each dose level, enables researchers to design robust protocols that balance efficacy investigation with tolerability considerations.
Key Implementation Steps
1. Protocol Design: Select the appropriate escalation strategy (full, moderate, or conservative) based on research objectives and population characteristics.
2. Quality Sourcing: Obtain high-purity, research-grade tirzepatide from reputable suppliers like PEPTIDE PRO that provide comprehensive documentation and quality assurance.
3. Infrastructure Preparation: Establish appropriate storage, reconstitution, and administration protocols to maintain compound integrity throughout the research period.
4. Monitoring Framework: Implement systematic assessment schedules that capture tolerability, safety, and efficacy parameters at each dose level.
5. Data Management: Develop comprehensive data collection systems that document dosing, administration, adverse events, and research outcomes for thorough analysis.
Next Steps for Researchers
Ready to incorporate tirzepatide into your research protocols? Consider these actionable steps:
🔬 Review Protocol Requirements: Assess your research objectives to determine the optimal escalation strategy and maintenance dose targets.
🔬 Evaluate Infrastructure: Ensure appropriate storage, reconstitution, and administration capabilities are in place before compound acquisition.
🔬 Source Quality Compounds: Explore PEPTIDE PRO’s tirzepatide formulations to identify the concentration that best suits your protocol needs.
🔬 Develop Monitoring Systems: Establish comprehensive assessment schedules and data collection frameworks before protocol initiation.
🔬 Consult Technical Support: For questions about product specifications, storage, or reconstitution, contact PEPTIDE PRO’s support team for expert guidance.
Final Considerations
The systematic approach outlined in this Tirzepatide Dosage Chart (All Doses + Timeline) guide reflects evidence-based protocols developed through extensive clinical research. By adhering to these established frameworks while maintaining the flexibility to optimize for specific research applications, investigators can maximize the value and reliability of tirzepatide research outcomes.
Remember that all tirzepatide products are strictly for research use only and not approved for human or animal consumption. Researchers should ensure full compliance with institutional review board requirements, regulatory guidelines, and ethical safety protocols when designing and implementing tirzepatide research studies.
With proper protocol design, quality compound sourcing, and systematic implementation of the dosing framework outlined in this guide, researchers can contribute valuable insights to the expanding body of knowledge surrounding this innovative dual incretin receptor agonist.
References
[1] Mounjaro (tirzepatide) Prescribing Information. Eli Lilly and Company. 2023.
[2] Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387(3):205-216.
[3] Zepbound (tirzepatide) Prescribing Information. Eli Lilly and Company. 2023.
[4] Frias JP, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385(6):503-515.
[5] FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes. U.S. Food and Drug Administration. May 2022.
[6] Rosenstock J, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143-155.
[7] Dahl D, et al. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022;327(6):534-545.
[8] Ludvik B, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583-598.
[9] Wilson JM, et al. Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program. Obesity (Silver Spring). 2023;31(1):96-110.
