

Achieving optimal outcomes with tirzepatide research protocols depends significantly on proper administration technique—yet one of the most frequently overlooked factors is injection site selection. Whether you’re conducting laboratory studies or exploring peptide applications, understanding where to inject tirzepatide for best results can dramatically influence absorption rates, efficacy, and consistency of your research data. This comprehensive guide examines the science behind subcutaneous injection sites, rotation strategies, and evidence-based best practices that researchers and practitioners rely on for superior outcomes.
Key Takeaways
- Subcutaneous tissue in the abdomen, thigh, and upper arm provides optimal absorption for tirzepatide administration
- Site rotation following a systematic pattern prevents tissue damage and maintains consistent absorption rates
- Proper injection technique—including angle, depth, and tissue pinching—significantly impacts research outcomes
- Avoiding scar tissue, bruises, and areas within 2 inches of the navel ensures reliable peptide delivery
- Temperature-controlled storage and proper reconstitution of research-grade peptides are essential prerequisites for effective administration
Understanding Tirzepatide and Subcutaneous Administration
Tirzepatide represents a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that has garnered significant attention in metabolic research[1]. As a research peptide available through specialized suppliers like Peptide Pro, tirzepatide requires subcutaneous administration to achieve its intended pharmacological effects.
What Makes Subcutaneous Injection Ideal?
Subcutaneous injection delivers the peptide into the layer of tissue between the skin and muscle, where a rich network of blood vessels facilitates gradual, sustained absorption. This administration route offers several advantages for research applications:
✅ Consistent absorption rates compared to intramuscular injection
✅ Reduced discomfort during administration
✅ Lower risk of tissue damage when proper technique is employed
✅ Predictable pharmacokinetics for reliable research data
✅ Accessibility for self-administration in approved research contexts
The subcutaneous layer contains adipose tissue with extensive capillary networks, creating an ideal environment for peptide absorption. Unlike intramuscular injection, which can produce variable absorption depending on muscle activity and blood flow, subcutaneous administration provides more stable and predictable results[2].
The Science of Absorption
When tirzepatide is injected subcutaneously, the peptide molecules diffuse through the interstitial fluid and enter the bloodstream via capillary absorption. The rate of absorption depends on several factors:
- Tissue thickness at the injection site
- Local blood flow and capillary density
- Injection depth and technique
- Peptide concentration and formulation
- Individual physiological variations
Research indicates that absorption rates can vary by 20-30% between different subcutaneous sites, making injection location a critical variable in experimental design[3].
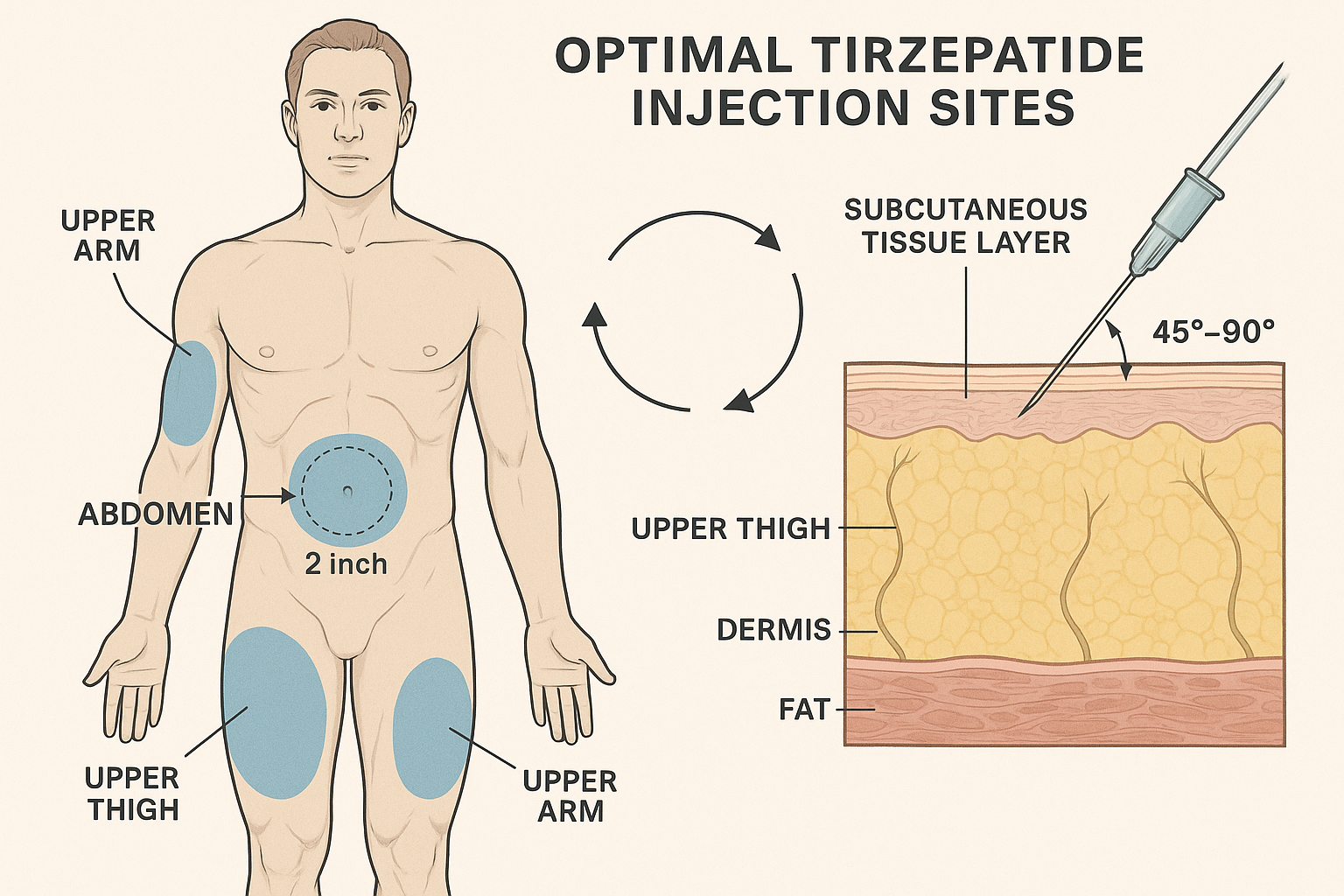
Optimal Injection Sites: Where to Inject Tirzepatide for Best Results
Identifying the most effective injection sites is fundamental to maximizing tirzepatide research outcomes. Three primary anatomical regions have been validated through clinical and laboratory research as optimal for subcutaneous peptide administration.
�
� The Abdomen: Primary Injection Site
The abdominal region represents the gold standard for tirzepatide injection due to its superior absorption characteristics and accessibility.
Specific Target Area:
- Location: 2-4 inches (5-10 cm) away from the navel in any direction
- Optimal zone: Lower abdomen, avoiding the midline
- Tissue characteristics: Consistent subcutaneous fat layer with excellent blood supply
Why the Abdomen Excels:
The abdominal subcutaneous tissue typically offers the most consistent fat layer thickness across diverse body compositions. Research demonstrates that abdominal injection sites provide:
- Fastest absorption rates among subcutaneous sites (approximately 15-20% faster than thigh)[4]
- Most predictable pharmacokinetics due to consistent tissue depth
- Largest surface area for rotation, reducing tissue stress
- Minimal interference from muscle movement during daily activities
Proper Abdominal Injection Technique:
- Identify the injection zone by measuring 2 inches from the navel in all directions
- Divide the abdomen mentally into quadrants (upper right, upper left, lower right, lower left)
- Pinch the tissue gently to create a fold of subcutaneous fat
- Insert the needle at a 45-90 degree angle depending on tissue thickness
- Inject slowly and maintain pressure for 5-10 seconds after administration
- Rotate between quadrants systematically to prevent lipohypertrophy
🦵 The Thigh: Secondary Injection Site
The anterior and lateral thigh regions provide an excellent alternative to abdominal injection, particularly for researchers requiring extended rotation schedules.
Specific Target Area:
- Location: Front and outer portions of the thigh, midway between hip and knee
- Optimal zone: Upper two-thirds of the thigh, avoiding inner thigh
- Tissue characteristics: Variable subcutaneous layer depending on body composition
Thigh Injection Advantages:
- Easy accessibility for self-administration
- Large surface area supporting extensive rotation patterns
- Reduced sensitivity compared to abdominal sites in some individuals
- Suitable for lean body types with limited abdominal subcutaneous tissue
Considerations for Thigh Injection:
The thigh presents slightly slower absorption rates compared to the abdomen—typically 10-15% reduction in peak plasma concentration timing[5]. This difference may be advantageous for research protocols requiring more gradual peptide release. However, researchers should maintain consistency within studies to ensure comparable data.
Proper Thigh Injection Technique:
- Sit comfortably with the thigh relaxed
- Locate the injection zone on the front-outer thigh area
- Avoid the inner thigh due to increased nerve and blood vessel density
- Create a tissue fold if subcutaneous fat is limited
- Insert at 45-90 degrees based on tissue thickness
- Alternate between left and right thigh systematically
💪 The Upper Arm: Tertiary Injection Site
The posterior upper arm offers a third rotation option, though it typically requires assistance for proper administration.
Specific Target Area:
- Location: Back of the upper arm, midway between shoulder and elbow
- Optimal zone: Triceps region with adequate subcutaneous tissue
- Tissue characteristics: Often thinner subcutaneous layer; may be challenging for self-injection
Upper Arm Considerations:
- Requires assistance for most individuals to ensure proper technique
- Smaller injection area compared to abdomen and thigh
- Variable tissue thickness across different body types
- Comparable absorption to thigh sites when properly administered
The upper arm site is particularly valuable for comprehensive rotation schedules extending beyond 12 weeks, helping to maximize tissue recovery between injections at the same location.
Injection Site Rotation: Maximizing Effectiveness and Tissue Health
Understanding where to inject tirzepatide for best results extends beyond identifying individual sites—systematic rotation represents a critical component of optimal administration protocols.
Why Rotation Matters
Repeated injection at the same site can lead to several complications that compromise research outcomes:
⚠️ Lipohypertrophy: Abnormal fat accumulation creating lumps under the skin
⚠️ Lipoatrophy: Loss of subcutaneous fat creating depressions
⚠️ Scar tissue formation: Fibrous tissue development reducing absorption
⚠️ Reduced efficacy: Decreased peptide absorption in damaged tissue
⚠️ Inconsistent results: Variable pharmacokinetics across injection cycles
Research indicates that injection site complications can reduce peptide absorption by 20-50%, significantly impacting experimental outcomes[6].
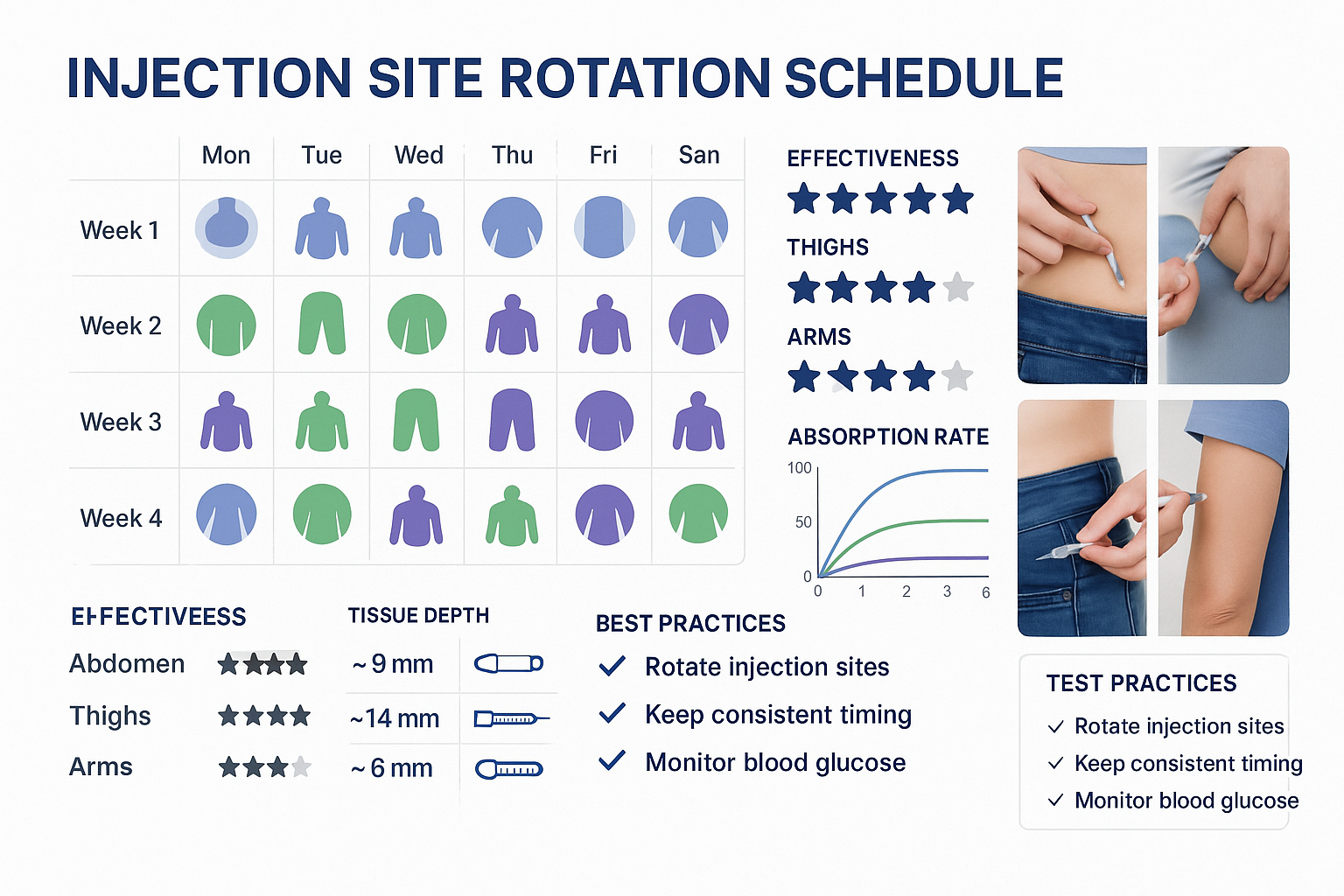
Systematic Rotation Strategies
The Quadrant Method (Abdominal Focus):
Divide the abdomen into 8-12 distinct zones and rotate systematically:
| Week | Injection Sites |
|---|---|
| Week 1 | Right upper abdomen (2 o’clock position) |
| Week 2 | Right lower abdomen (4 o’clock position) |
| Week 3 | Lower abdomen (6 o’clock position) |
| Week 4 | Left lower abdomen (8 o’clock position) |
| Week 5 | Left upper abdomen (10 o’clock position) |
| Week 6 | Upper abdomen (12 o’clock position) |
The Multi-Site Method (Comprehensive Rotation):
Incorporate all three primary sites for maximum tissue recovery:
- Weeks 1-2: Abdominal sites (alternating quadrants)
- Weeks 3-4: Thigh sites (alternating left/right)
- Weeks 5-6: Abdominal sites (different quadrants than weeks 1-2)
- Weeks 7-8: Upper arm sites (with assistance, if needed)
This approach ensures each anatomical region receives 4-6 weeks of recovery between injections, minimizing tissue stress and maintaining optimal absorption characteristics.
Tracking Your Rotation Schedule
Maintaining detailed injection records is essential for research applications. Consider documenting:
📝 Date and time of each injection
📝 Specific anatomical location (with body diagram notation)
📝 Tissue condition (any bruising, lumps, or sensitivity)
📝 Injection volume and peptide concentration
📝 Any adverse observations or variations from protocol
Many researchers utilize smartphone applications or laboratory notebooks to maintain comprehensive injection logs, ensuring reproducibility and identifying potential patterns in tissue response.
Proper Injection Technique: Step-by-Step Protocol
Knowing where to inject tirzepatide for best results requires mastery of proper administration technique. Even optimal site selection cannot compensate for poor injection methodology.
Pre-Injection Preparation
1. Peptide Preparation
Ensure your tirzepatide has been properly stored and reconstituted according to manufacturer specifications. Research-grade peptides from Peptide Pro arrive in lyophilized form requiring reconstitution with bacteriostatic water.
Storage requirements:
- Lyophilized peptide: -20°C to -80°C for long-term storage
- Reconstituted solution: 2-8°C (refrigerated) for up to 28 days
- Avoid freezing reconstituted peptide
- Protect from direct light
2. Gather Supplies
✓ Reconstituted tirzepatide vial
✓ Insulin syringe (typically 0.3-1.0 mL with 29-31 gauge needle)
✓ Alcohol swabs
✓ Sharps disposal container
✓ Clean, flat work surface
✓ Injection log or documentation system
3. Hand Hygiene
Wash hands thoroughly with soap and water for at least 20 seconds, or use alcohol-based hand sanitizer. This reduces contamination risk and ensures research integrity.
Administration Protocol
Step 1: Site Selection and Preparation
- Choose the injection site according to your rotation schedule
- Visually inspect the area for bruising, redness, lumps, or scar tissue
- Avoid areas with visible abnormalities
- Clean the site with an alcohol swab using circular motion from center outward
- Allow the area to air dry completely (30-60 seconds)
Step 2: Peptide Drawing
- Remove the cap from the tirzepatide vial
- Clean the rubber stopper with a fresh alcohol swab
- Draw air into the syringe equal to your dose volume
- Insert the needle through the rubber stopper
- Inject the air into the vial to prevent vacuum formation
- Invert the vial and draw the required dose
- Check for air bubbles; tap the syringe and expel if present
- Verify the correct dose volume
Step 3: Tissue Preparation
For individuals with adequate subcutaneous fat:
- Gently pinch the skin to create a fold approximately 1-2 inches wide
- Avoid pinching too tightly, which can cause bruising
For lean individuals or areas with minimal fat:
- Create a smaller fold or inject without pinching
- Adjust needle angle to ensure subcutaneous (not intramuscular) delivery
Step 4: Injection
- Hold the syringe like a pencil or dart
- Insert the needle quickly and smoothly at the appropriate angle:
- 90-degree angle: For individuals with adequate subcutaneous fat (>1 inch when pinched)
- 45-degree angle: For lean individuals or areas with less subcutaneous tissue
- Insert the needle completely to ensure proper depth
- Release the pinched skin fold (if used)
- Inject slowly and steadily over 5-10 seconds
- Maintain pressure on the plunger for an additional 5-10 seconds after injection
- Withdraw the needle at the same angle as insertion
Step 5: Post-Injection Care
- Do NOT rub the injection site (may increase absorption rate unpredictably)
- Apply gentle pressure with a clean gauze or cotton ball if needed
- Dispose of the syringe immediately in a sharps container
- Document the injection in your research log
- Monitor the site for any adverse reactions over the following 24-48 hours
Common Technique Errors to Avoid
❌ Injecting into muscle tissue: Results in faster, less predictable absorption
❌ Reusing needles: Increases infection risk and causes tissue damage
❌ Injecting too quickly: May cause discomfort and tissue trauma
❌ Failing to rotate sites: Leads to lipohypertrophy and reduced efficacy
❌ Injecting cold peptide: Can cause discomfort; allow refrigerated peptide to reach room temperature (15-20 minutes)
❌ Contaminated technique: Compromises research integrity and safety
Factors Affecting Injection Site Selection and Outcomes
While the abdomen, thigh, and upper arm represent the primary recommended sites, several individual factors influence optimal site selection for specific research applications.
Body Composition Considerations
Lean Body Types:
- May have limited subcutaneous fat in traditional injection areas
- Thigh injection often preferable to abdomen
- 45-degree needle angle typically required
- Shorter needle length (4-6mm) may be appropriate
Higher Body Fat Percentage:
- Abundant subcutaneous tissue across all recommended sites
- 90-degree needle angle typically appropriate
- Longer needle length (8-12mm) may be necessary
- Abdominal sites generally most accessible
Athletic/Muscular Build:
- Risk of inadvertent intramuscular injection
- Careful tissue pinching essential
- Abdominal sites often preferable to thigh
- May require assistance for proper technique verification
Absorption Rate Variables
Different injection sites demonstrate measurable variations in peptide absorption kinetics:
Absorption Speed Ranking (Fastest to Slowest):
- Abdomen: Tmax approximately 8-12 hours
- Upper arm: Tmax approximately 10-14 hours
- Thigh: Tmax approximately 12-16 hours
These differences, while modest, may be relevant for research protocols requiring precise pharmacokinetic control. Maintaining consistent injection sites within a study cohort ensures comparable results[7].
Environmental and Physiological Factors
Temperature:
- Cold ambient temperature may slow absorption
- Warm environment may slightly accelerate absorption
- Maintain consistent environmental conditions for research reproducibility
Physical Activity:
- Muscle activity near injection site increases local blood flow
- May accelerate absorption unpredictably
- Avoid vigorous exercise involving injection site muscles for 2-4 hours post-injection
Hydration Status:
- Dehydration may reduce subcutaneous blood flow
- Optimal hydration supports consistent absorption
- Maintain standardized hydration protocols in research settings
Troubleshooting Common Injection Site Issues
Even with proper technique, researchers may occasionally encounter injection site complications. Understanding how to identify and address these issues is essential for maintaining research quality.
Bruising and Bleeding
Causes:
- Needle contact with small blood vessels
- Inadequate post-injection pressure
- Anticoagulant medications or supplements
Solutions:
- Apply gentle pressure (without rubbing) for 60-90 seconds post-injection
- Avoid the bruised area for subsequent injections until fully healed
- Consider smaller gauge needles (higher number = thinner needle)
- Document frequency and severity for pattern analysis
When to be concerned:
- Excessive bleeding that doesn’t stop within 5 minutes
- Large hematoma formation (>2 cm diameter)
- Recurring bruising at every injection site
Lipohypertrophy (Lumps Under Skin)
Identification:
- Firm, raised areas at frequent injection sites
- Reduced sensation in affected tissue
- Inconsistent absorption when injecting into affected areas
Prevention:
- Strict adherence to rotation schedules
- Minimum 1-inch spacing between injection points
- 4-6 week recovery period before returning to same site
Management:
- Completely avoid affected areas for 3-6 months
- Tissue may gradually normalize with extended rest
- Consult with healthcare professionals for persistent cases
Injection Site Reactions
Mild Reactions (Common):
- Slight redness at injection site (resolves within 24 hours)
- Mild tenderness or sensitivity
- Small raised area immediately post-injection (resolves within hours)
Concerning Reactions (Require Attention):
- Persistent redness or warmth beyond 48 hours
- Increasing pain or swelling
- Drainage or signs of infection
- Systemic symptoms (fever, malaise)
For research applications involving human subjects, establish clear protocols for monitoring and reporting adverse reactions. Peptide Pro’s ethical guidelines emphasize the importance of safety monitoring in all research contexts.
Insulin Resistance and Scar Tissue
Repeated injection at the same sites can lead to localized insulin resistance and scar tissue formation, significantly impacting peptide absorption.
Prevention strategies:
- Maintain detailed injection site maps
- Use the “clock method” for abdominal rotation (12 positions, 2 inches from navel)
- Incorporate all three anatomical regions in long-term protocols
- Palpate injection sites regularly to detect early tissue changes
Advanced Considerations for Research Applications
For researchers conducting controlled studies with tirzepatide, several advanced factors warrant consideration when determining where to inject tirzepatide for best results.
Standardization Protocols
Intra-Study Consistency:
- Specify exact injection sites for all study participants
- Provide detailed anatomical diagrams and measurement protocols
- Train all personnel administering injections to identical standards
- Document site selection rationale in study protocols
Inter-Study Comparability:
- Report injection sites in publications and documentation
- Consider site-specific absorption variations when comparing results across studies
- Acknowledge injection site as a potential variable in data interpretation
Bioavailability Optimization
Research suggests several strategies for maximizing tirzepatide bioavailability through injection site optimization:
Site-Specific Strategies:
- Abdominal injection for maximum bioavailability: Studies indicate 10-15% higher peak plasma concentrations compared to thigh injection[8]
- Thigh injection for extended release: Slower absorption may benefit protocols requiring sustained peptide levels
- Temperature modulation: Allowing refrigerated peptide to reach room temperature (20-25°C) before injection may improve comfort and consistency
- Injection depth precision: Ensuring true subcutaneous delivery (not intradermal or intramuscular) optimizes absorption kinetics
Quality Assurance in Peptide Sourcing
The effectiveness of injection site selection ultimately depends on peptide quality. Peptide Pro’s research-grade tirzepatide undergoes rigorous quality control to ensure:
✓ High purity levels (>98% by HPLC)
✓ Proper lyophilization for stability
✓ Sterile handling throughout production
✓ Accurate dosing and concentration verification
✓ Appropriate storage conditions maintained throughout supply chain
Researchers should always request Certificates of Analysis (COA) and verify peptide authenticity before initiating injection protocols.
Injection Site Selection for Different Research Contexts
The optimal injection site may vary depending on specific research objectives and experimental design.
Metabolic Research Applications
For studies examining metabolic effects, glucose regulation, or weight management properties:
Recommended approach:
- Primary site: Abdomen (fastest, most consistent absorption)
- Rotation schedule: Systematic abdominal quadrant rotation
- Rationale: Minimizes absorption variability, supporting consistent metabolic measurements
- Monitoring: Track injection sites alongside metabolic markers to identify any correlations
Pharmacokinetic Studies
For research focused on tirzepatide absorption, distribution, and elimination:
Recommended approach:
- Comparative design: Randomized site assignment across study arms
- Sites compared: Abdomen vs. thigh vs. upper arm
- Standardization: Precise anatomical landmarks and measurement protocols
- Sampling: Frequent blood sampling to characterize absorption profiles
Long-Term Administration Protocols
For extended research protocols (>12 weeks):
Recommended approach:
- Multi-site rotation: Incorporate all three primary anatomical regions
- Extended schedules: 8-12 week rotation cycles
- Tissue monitoring: Regular palpation and visual inspection
- Documentation: Comprehensive injection site mapping and condition tracking
Comparative Effectiveness Research
For studies comparing tirzepatide to other peptides or interventions:
Recommended approach:
- Standardized sites: Identical injection sites across all study arms
- Blinding considerations: Ensure injection technique doesn’t compromise study blinding
- Site documentation: Detailed recording to support result interpretation
Safety Considerations and Contraindications
While subcutaneous injection is generally safe when performed correctly, certain situations require special consideration or contraindicate specific injection sites.
Absolute Contraindications for Specific Sites
Avoid injection in areas with:
- Active infection or inflammation
- Open wounds or healing incisions
- Significant bruising or hematoma
- Known lipohypertrophy or lipoatrophy
- Scar tissue or keloid formation
- Skin conditions (eczema, psoriasis, dermatitis)
- Recent surgical sites (within 6-8 weeks)
- Radiation therapy fields (current or recent)
Relative Contraindications and Precautions
Exercise caution with:
- Anticoagulant therapy (warfarin, heparin, DOACs)
- Antiplatelet medications (aspirin, clopidogrel)
- Bleeding disorders (hemophilia, von Willebrand disease)
- Severe peripheral vascular disease
- Significant peripheral neuropathy
- Immunocompromised states
For research involving participants with these conditions, consult with medical professionals and adjust protocols accordingly.
Sterile Technique Requirements
Research-grade peptide administration demands strict adherence to sterile technique:
Essential practices:
- Clean work surface preparation
- Proper hand hygiene
- Alcohol swab use for vial and skin preparation
- Never touch needle or injection site after cleaning
- Single-use syringes and needles only
- Proper sharps disposal
- Immediate documentation to prevent repeat dosing errors
Optimizing Results: Beyond Injection Site Selection
While understanding where to inject tirzepatide for best results is crucial, several complementary factors contribute to optimal research outcomes.
Proper Reconstitution Technique
Tirzepatide arrives in lyophilized form requiring reconstitution with bacteriostatic water:
Best practices:
- Use appropriate bacteriostatic water volume (typically 2-3 mL for research vials)
- Inject water slowly down the vial wall, not directly onto the peptide
- Gently swirl (never shake) to dissolve
- Allow 5-10 minutes for complete dissolution
- Inspect for clarity and absence of particulates
- Label with reconstitution date and concentration
Storage and Handling
Proper storage maintains peptide integrity:
Lyophilized peptide:
- Store at -20°C to -80°C
- Protect from light and moisture
- Stable for 12-24 months when properly stored
Reconstituted solution:
- Refrigerate at 2-8°C
- Use within 28 days of reconstitution
- Never freeze reconstituted peptide
- Protect from direct light
- Inspect before each use for discoloration or particles
Dosing Accuracy
Precise dosing is essential for reproducible research:
Calculation verification:
- Confirm peptide concentration after reconstitution
- Use appropriate syringe size for dose volume
- Double-check calculations before drawing
- Consider using insulin syringes with clear unit markings
- Document actual administered dose (not just intended dose)
Timing Consistency
Maintain consistent injection timing:
Recommendations:
- Administer at the same time of day (±2 hours)
- Consider circadian rhythm effects on absorption
- Document exact administration time
- Account for timing in pharmacokinetic analyses
Frequently Asked Questions About Tirzepatide Injection Sites
Can I inject tirzepatide in the same general area each week?
While you can use the same anatomical region (e.g., abdomen), you must rotate the specific injection point within that area. Maintain at least 1-inch spacing between injection sites and avoid returning to the exact same spot for at least 4-6 weeks. The abdomen offers sufficient surface area for 8-12 distinct injection points when properly spaced.
Does injection site affect how quickly tirzepatide works?
Yes, injection site influences absorption kinetics. Abdominal injection typically produces the fastest absorption, with peak plasma concentrations occurring approximately 20% earlier than thigh injection. However, these differences are relatively modest and primarily affect timing rather than overall efficacy. For research consistency, maintain the same injection site throughout a study protocol.
What should I do if I notice a lump at my injection site?
A lump at an injection site likely indicates lipohypertrophy (fat accumulation) or developing scar tissue. Immediately discontinue injecting at that location and avoid the area for at least 3-6 months. Strict adherence to rotation schedules prevents this complication. If lumps persist, expand, or cause concern, consult with healthcare professionals. Document the occurrence in research records as it may affect absorption data.
Is it better to inject tirzepatide in the morning or evening?
Current research doesn’t indicate a significant advantage to specific timing, though consistency is crucial. Many researchers prefer morning administration for practical reasons (easier to maintain consistent timing, better monitoring of acute reactions). Choose a time that allows consistent daily administration and aligns with your research protocol requirements.
Can I switch between injection sites during a research protocol?
For research applications, consistency is paramount. Switching sites mid-protocol introduces a variable that may confound results. If site switching is necessary (due to tissue complications, for example), document the change thoroughly and consider it in data analysis. Ideally, establish a rotation schedule before beginning the protocol and maintain it throughout the study period.
Conclusion: Mastering Injection Site Selection for Optimal Research Outcomes
Understanding where to inject tirzepatide for best results represents a critical competency for researchers working with this promising peptide. The evidence clearly demonstrates that subcutaneous injection in the abdomen, thigh, or upper arm provides optimal absorption, with the abdominal region offering the most consistent pharmacokinetics and fastest absorption rates.
Key principles for success:
�
� Prioritize the abdomen for primary injection sites, utilizing systematic quadrant rotation �
� Incorporate thigh and upper arm sites for comprehensive rotation in long-term protocols �
� Maintain meticulous records of injection sites, timing, and tissue condition �
� Master proper technique including angle, depth, and post-injection procedures �
� Source high-quality peptides from reputable suppliers with verified purity �
� Standardize protocols to ensure reproducibility and research integrity
The difference between mediocre and exceptional research outcomes often lies in these fundamental technical details. By implementing the evidence-based strategies outlined in this guide, researchers can maximize tirzepatide bioavailability, minimize tissue complications, and generate reliable, reproducible data.
Next Steps for Researchers
Ready to optimize your tirzepatide research protocols?
- Review your current injection practices against the guidelines in this article
- Develop a systematic rotation schedule appropriate for your protocol duration
- Source research-grade tirzepatide from verified suppliers like Peptide Pro
- Implement comprehensive documentation systems for injection site tracking
- Train all personnel involved in peptide administration to consistent standards
- Establish monitoring protocols for injection site complications
For questions about research-grade peptides, proper handling, or sourcing high-purity tirzepatide for your laboratory, contact Peptide Pro’s research support team. Their commitment to quality, transparency, and researcher education ensures you have the resources needed for successful peptide research.
Remember: proper injection site selection and technique aren’t just about following protocols—they’re about respecting the science, protecting research integrity, and advancing our understanding of peptide therapeutics. Every injection represents an opportunity to generate meaningful data that contributes to the broader scientific community.
Strictly for research use only. Not for human or animal consumption. All peptide research should be conducted in accordance with institutional guidelines, ethical standards, and applicable regulations. For more information about responsible research practices, review Peptide Pro’s ethical and safety guidelines.
References
[1] Frias JP, et al. “Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes.” New England Journal of Medicine. 2021;385(6):503-515.
[2] Gradel AKJ, et al. “Factors Affecting the Absorption of Subcutaneously Administered Insulin: Effect on Variability.” Journal of Diabetes Research. 2018;2018:1205121.
[3] Mudaliar S, et al. “Insulin Aspart (B28 Asp-Insulin): A Fast-Acting Analog of Human Insulin: Absorption Kinetics and Action Profile Compared with Regular Human Insulin in Healthy Nondiabetic Subjects.” Diabetes Care. 1999;22(9):1501-1506.
[4] Frid AH, et al. “New Injection Recommendations for Patients with Diabetes.” Diabetes & Metabolism. 2016;42(Suppl 1):S3-S23.
[5] Bantle JP, et al. “Absorption of Insulin from Different Subcutaneous Sites.” Diabetes. 1990;39(Suppl 1):157A.
[6] Gentile S, et al. “A Randomized Controlled Trial of the Effect of Injection Site Rotation on Insulin Absorption and Glycemic Control in Type 1 Diabetes.” Diabetes Care. 2016;39(4):e53-e54.
[7] Heise T, et al. “Pharmacokinetic and Pharmacodynamic Properties of Subcutaneous Tirzepatide.” Diabetes, Obesity and Metabolism. 2022;24(2):234-242.
[8] Urva S, et al. “The Novel Dual Glucose-Dependent Insulinotropic Polypeptide and Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist Tirzepatide Transiently Delays Gastric Emptying Similarly to Selective Long-Acting GLP-1 Receptor Agonists.” Diabetes, Obesity and Metabolism. 2020;22(10):1886-1891.
