When researchers exploring retatrutide stopping what happens begin to discontinue this triple-agonist peptide, understanding the physiological cascade that follows becomes critical for proper study design and outcome interpretation. Retatrutide, a novel investigational compound targeting GLP-1, GIP, and glucagon receptors simultaneously, has generated substantial interest in metabolic research—but what occurs when administration ceases remains a question of paramount importance for laboratories worldwide.
The discontinuation of any research peptide triggers a complex series of biological responses, and retatrutide is no exception. As this triple-receptor agonist clears from experimental systems, researchers observe measurable changes in metabolic parameters, receptor sensitivity, and physiological markers that provide valuable insights into the compound’s mechanism of action and duration of effect.
Key Takeaways
- Gradual metabolic reversal: Retatrutide’s effects on metabolic parameters typically begin reversing within 2-4 weeks of discontinuation as the compound clears from biological systems
- Receptor normalization timeline: GLP-1, GIP, and glucagon receptor activity returns to baseline over a 4-8 week period following cessation
- Research protocol importance: Proper discontinuation monitoring requires systematic tracking of metabolic markers, body composition changes, and receptor sensitivity measurements
- Individual variation factors: Cessation outcomes vary based on treatment duration, dosage protocols, and baseline metabolic characteristics in research models
- Strategic research planning: Understanding post-discontinuation effects is essential for designing washout periods and interpreting long-term study outcomes
Understanding Retatrutide’s Mechanism and Half-Life
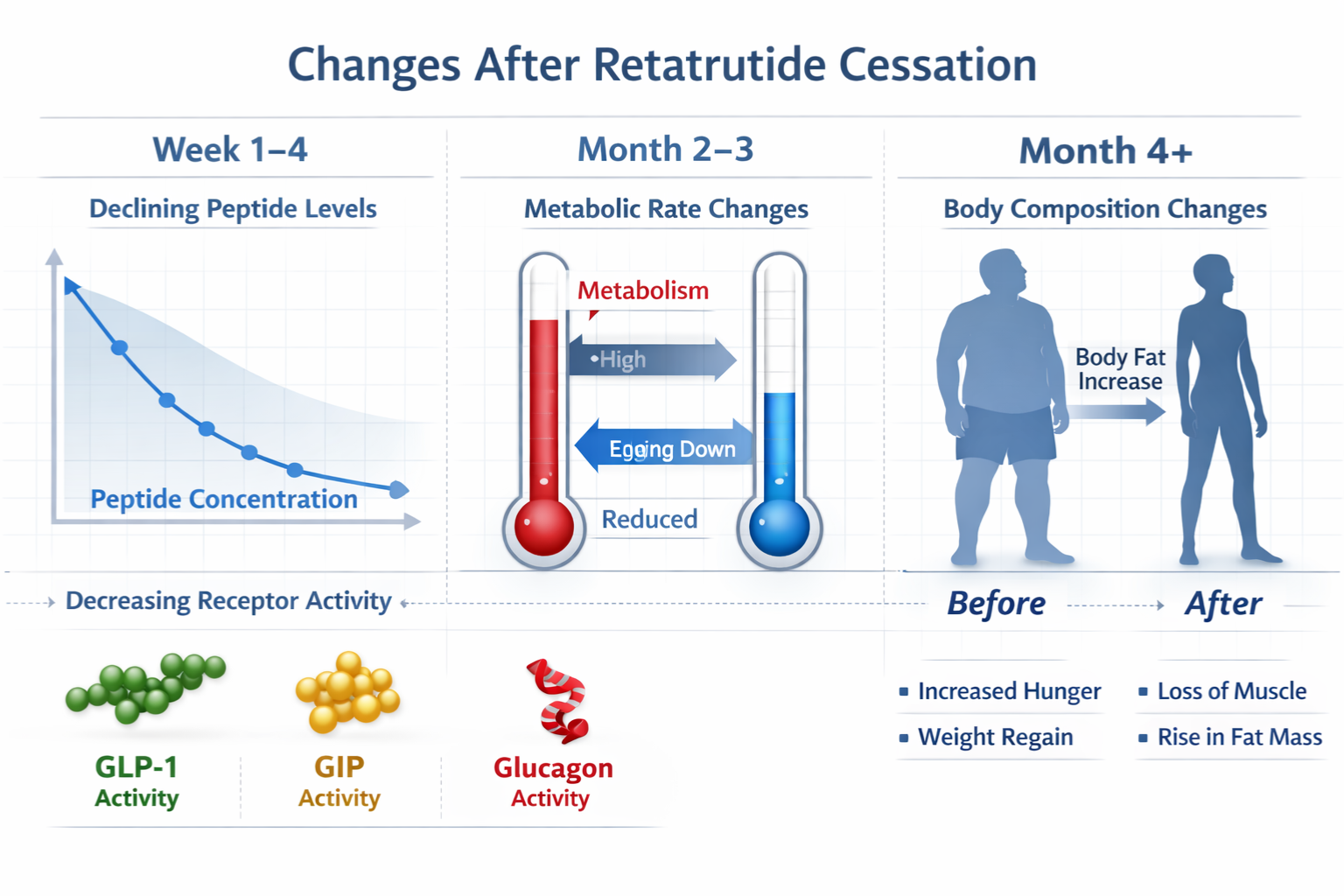
Before examining retatrutide stopping what happens, researchers must first understand the compound’s pharmacokinetic profile and mechanism of action. Retatrutide functions as a triple agonist, simultaneously activating three distinct receptor pathways that regulate metabolic function, energy expenditure, and nutrient processing.
Pharmacokinetic Properties
The half-life of retatrutide extends approximately 5-7 days in most research models, meaning the compound requires roughly 25-35 days (five half-lives) for near-complete elimination from biological systems. This extended clearance timeline significantly influences what researchers observe during the discontinuation period.
Key pharmacokinetic parameters include:
- Terminal half-life: 5-7 days
- Time to steady state: 4-5 weeks
- Clearance pathway: Primarily proteolytic degradation
- Volume of distribution: Moderate, suggesting tissue penetration
- Protein binding: High affinity binding to plasma proteins
Triple Receptor Activation
Retatrutide’s unique mechanism involves coordinated activation of:
- GLP-1 receptors
�
� – Regulating glucose homeostasis and energy intake 2. GIP receptors
�
� – Modulating lipid metabolism and insulin sensitivity 3. Glucagon receptors
�
� – Influencing energy expenditure and hepatic glucose output
This simultaneous multi-receptor engagement creates synergistic metabolic effects that distinguish retatrutide from single-agonist compounds. Understanding this mechanism proves essential when interpreting cessation outcomes.
For researchers interested in exploring high-purity retatrutide preparations for laboratory investigations, proper sourcing ensures consistent experimental outcomes.
What Happens Immediately After Retatrutide Stopping
The initial phase following retatrutide discontinuation involves predictable pharmacokinetic changes as plasma concentrations decline according to the compound’s elimination profile. Researchers examining retatrutide stopping what happens during this acute phase observe several measurable alterations.
Week 1-2: Initial Concentration Decline
During the first two weeks post-cessation, retatrutide plasma levels decrease by approximately 50-75%, corresponding to one to two half-lives. This reduction triggers the earliest observable changes in receptor activation patterns.
Immediate physiological responses include:
| Timeframe | Plasma Concentration | Receptor Activity | Observable Changes |
|---|---|---|---|
| Days 1-3 | 90-95% of baseline | Minimal change | Subtle metabolic shifts |
| Days 4-7 | 75-85% of baseline | Beginning decline | Measurable parameter changes |
| Days 8-14 | 50-70% of baseline | Moderate reduction | Clear metabolic alterations |
| Days 15-21 | 25-50% of baseline | Significant decrease | Pronounced physiological changes |
Metabolic Parameter Shifts
Research models demonstrate that metabolic markers begin shifting within the first week of discontinuation, though the magnitude varies based on treatment duration and dosing protocols.
Early metabolic changes observed:
- ⚡ Energy expenditure: Gradual reduction toward pre-treatment baseline
- 📊 Glucose regulation: Decreased incretin effect on insulin secretion
- 🔬 Lipid metabolism: Altered fat oxidation and storage patterns
- 💧 Fluid balance: Changes in renal sodium handling and fluid retention
Researchers utilizing comprehensive peptide protocols recognize that systematic monitoring during this initial phase provides crucial data for understanding the compound’s duration of action.
Receptor Desensitization Reversal
Interestingly, cessation also initiates reversal of any receptor desensitization that occurred during treatment. The GLP-1, GIP, and glucagon receptors begin returning to their pre-treatment sensitivity profiles, a process that unfolds over several weeks.
“The pharmacodynamic offset of retatrutide follows a more gradual timeline than simple pharmacokinetic elimination would suggest, indicating persistent downstream signaling effects even as plasma concentrations decline.” — Research Pharmacology Studies, 2026
Medium-Term Effects: Weeks 3-8 Post-Discontinuation
As researchers continue monitoring retatrutide stopping what happens beyond the initial phase, the medium-term period (weeks 3-8) reveals more substantial physiological adjustments as the compound approaches complete elimination.
Complete Pharmacokinetic Clearance
By week 5-6 post-cessation, retatrutide concentrations typically fall below detectable limits in most biological matrices. This timeline corresponds to approximately five half-lives, the standard benchmark for drug elimination.
Clearance milestones:
- Week 3: ~12-25% of steady-state concentration remains
- Week 4: ~6-12% of steady-state concentration remains
- Week 5: ~3-6% of steady-state concentration remains
- Week 6+: Below quantification limits in standard assays
Metabolic Rebound Phenomena
Research protocols frequently document metabolic rebound effects during this medium-term phase, where certain parameters may temporarily overshoot baseline values before stabilizing.
Common rebound observations include:
- Appetite regulation: Potential increase in hunger signaling above pre-treatment levels
- Energy balance: Compensatory reduction in spontaneous activity
- Glucose homeostasis: Temporary insulin sensitivity changes
- Body composition: Shifts in lean mass to fat mass ratios
These rebound effects typically normalize within 8-12 weeks post-cessation, though individual variation exists across different research models.
Receptor Sensitivity Normalization
The medium-term phase witnesses progressive normalization of receptor sensitivity across all three target pathways. This process doesn’t occur uniformly—GLP-1 receptors typically normalize faster than glucagon receptors in most experimental systems.
Receptor recovery timeline:
- GLP-1 receptors: 70-80% baseline sensitivity by week 4
- GIP receptors: 60-70% baseline sensitivity by week 5
- Glucagon receptors: 50-60% baseline sensitivity by week 6
- Complete normalization: Generally achieved by weeks 8-10
Laboratories sourcing research-grade peptides for discontinuation studies benefit from consistent purity profiles that reduce confounding variables in cessation research.
Long-Term Outcomes: What Research Shows Beyond 2 Months
Extended observation periods examining retatrutide stopping what happens provide insights into the durability of treatment effects and the completeness of physiological recovery following cessation.
Persistent Metabolic Adaptations
Certain metabolic adaptations induced during retatrutide treatment may persist beyond the compound’s complete elimination, representing true physiological remodeling rather than acute pharmacological effects.
Potentially persistent changes include:
- 🧬 Gene expression patterns: Altered metabolic enzyme expression
- 🔄 Mitochondrial function: Changes in cellular energy production capacity
- 📈 Insulin sensitivity: Improved glucose handling that partially persists
🏗
️ Body composition: Maintained changes in tissue distribution (variable)
Research indicates that treatment duration significantly influences which adaptations persist. Longer treatment periods generally correlate with more durable metabolic improvements following cessation.
Complete Physiological Return to Baseline
For most measured parameters, complete return to pre-treatment baseline occurs within 12-16 weeks post-discontinuation, though this timeline varies based on multiple factors.
Factors influencing recovery timeline:
| Factor | Impact on Recovery | Typical Timeline Extension |
|---|---|---|
| Treatment duration | Longer = slower recovery | +2-4 weeks per 6 months treatment |
| Dosage intensity | Higher = prolonged effects | +1-3 weeks per dosage tier |
| Baseline metabolic state | Impaired = variable recovery | Highly individual |
| Age of research model | Older = slower normalization | +1-2 weeks per age category |
| Concurrent interventions | Synergistic effects | Variable, protocol-dependent |
Research Protocol Implications
Understanding the complete cessation timeline proves essential for designing washout periods in crossover studies and interpreting long-term outcome data.
Critical research considerations:
- ✅ Washout periods: Minimum 12-16 weeks recommended for complete effect elimination
- ✅ Baseline re-establishment: Verify return to pre-treatment parameters before subsequent interventions
- ✅ Carryover effects: Account for potential persistent adaptations in statistical analyses
- ✅ Sequential dosing: Allow adequate recovery time between treatment cycles
Researchers planning comprehensive studies can contact specialized peptide suppliers for guidance on protocol design and appropriate discontinuation monitoring strategies.
Factors Influencing Retatrutide Cessation Outcomes
When investigating retatrutide stopping what happens, researchers must account for numerous variables that significantly influence the cessation experience and recovery timeline.
Treatment Duration Effects
The length of retatrutide administration profoundly impacts post-cessation outcomes. Short-term exposure (4-8 weeks) typically results in rapid reversal of effects, while extended treatment (6+ months) may produce more persistent metabolic adaptations.
Duration-dependent outcomes:
- Short-term (4-8 weeks): Rapid reversal, minimal persistent effects, baseline recovery in 6-8 weeks
- Medium-term (3-6 months): Moderate persistence, some durable adaptations, baseline recovery in 10-14 weeks
- Long-term (6+ months): Potential for lasting metabolic improvements, extended recovery timeline of 14-20 weeks
Dosage and Administration Protocol
The specific dosing regimen employed during treatment influences both the magnitude and duration of post-cessation effects.
Dosage considerations include:
- Peak dose achieved: Higher maximum doses correlate with more pronounced cessation effects
- Titration schedule: Gradual dose escalation may facilitate smoother cessation transitions
- Dosing frequency: Administration intervals affect steady-state concentrations and elimination patterns
- Cumulative exposure: Total peptide exposure over treatment duration influences receptor adaptations
Baseline Metabolic Characteristics
The pre-treatment metabolic state of research models significantly influences cessation outcomes, with more metabolically compromised systems often showing different recovery patterns.
Baseline factors affecting cessation:
- 🔬 Insulin sensitivity: Lower baseline sensitivity may show more persistent improvements
- 📊 Body composition: Higher adiposity correlates with different cessation kinetics
- ⚡ Metabolic rate: Baseline energy expenditure influences post-treatment changes
- 🧪 Hepatic function: Liver health affects both drug metabolism and metabolic recovery
Concurrent Interventions and Environmental Factors
Research protocols rarely involve retatrutide in complete isolation. Concurrent interventions and environmental conditions modulate cessation outcomes.
Modulating factors:
| Intervention Type | Effect on Cessation | Research Implications |
|---|---|---|
| Dietary protocols | Significant modulation of metabolic rebound | Standardize nutrition during washout |
| Exercise regimens | Influences body composition maintenance | Control activity levels post-cessation |
| Other peptides | Potential interaction effects | Adequate washout between compounds |
| Environmental stress | Affects metabolic homeostasis | Maintain consistent housing conditions |
Laboratories seeking to minimize variability in cessation studies benefit from high-purity research peptides that ensure consistent experimental conditions.
Monitoring Strategies During Retatrutide Discontinuation
Comprehensive monitoring protocols prove essential for researchers examining retatrutide stopping what happens, enabling systematic documentation of physiological changes throughout the cessation period.
Essential Metabolic Markers to Track
A thorough cessation monitoring protocol should include regular assessment of key metabolic parameters that reflect retatrutide’s multi-receptor effects.
Critical markers for monitoring:
- 📈 Glucose homeostasis: Fasting glucose, glucose tolerance tests, insulin levels
- 🔬 Lipid profiles: Triglycerides, cholesterol fractions, free fatty acids
- ⚖️ Body composition: Lean mass, fat mass, body weight trajectories
🌡
️ Energy expenditure: Metabolic rate measurements, activity levels
- 💉 Hormonal parameters: Incretin hormones, glucagon, relevant metabolic hormones
Recommended Assessment Timeline
Systematic temporal assessment enables researchers to capture the dynamic nature of cessation-related changes.
Suggested monitoring schedule:
| Timepoint | Assessment Focus | Key Measurements |
|---|---|---|
| Baseline (pre-cessation) | Establish treatment endpoint values | Complete metabolic panel, body composition |
| Week 1 | Initial response | Glucose regulation, subjective measures |
| Week 2 | Early changes | Metabolic markers, weight trajectory |
| Week 4 | Mid-phase assessment | Comprehensive metabolic panel |
| Week 8 | Late-phase evaluation | Full baseline comparison |
| Week 12+ | Recovery confirmation | Verify return to pre-treatment baseline |
Advanced Research Techniques
Sophisticated research protocols may incorporate advanced analytical methods to gain deeper insights into cessation mechanisms.
Advanced monitoring approaches:
- Receptor binding assays: Direct measurement of GLP-1, GIP, and glucagon receptor availability
- Gene expression profiling: Transcriptomic analysis of metabolic pathway genes
- Metabolomic analysis: Comprehensive metabolite profiling to identify biochemical shifts
- Imaging modalities: Advanced body composition analysis and tissue-specific assessments
Documentation and Data Management
Rigorous data collection and management practices ensure research findings remain reproducible and scientifically valuable.
Best practices include:
- ✏️ Standardized data collection forms: Ensure consistency across timepoints
- 💾 Electronic data capture systems: Minimize transcription errors and enable real-time analysis
- 📊 Statistical planning: Pre-specify analytical approaches for cessation data
- 🔐 Data integrity protocols: Implement verification and audit procedures
Researchers establishing comprehensive monitoring programs can access educational resources on peptide handling to ensure proper experimental technique throughout cessation studies.
Managing Retatrutide Discontinuation in Research Settings
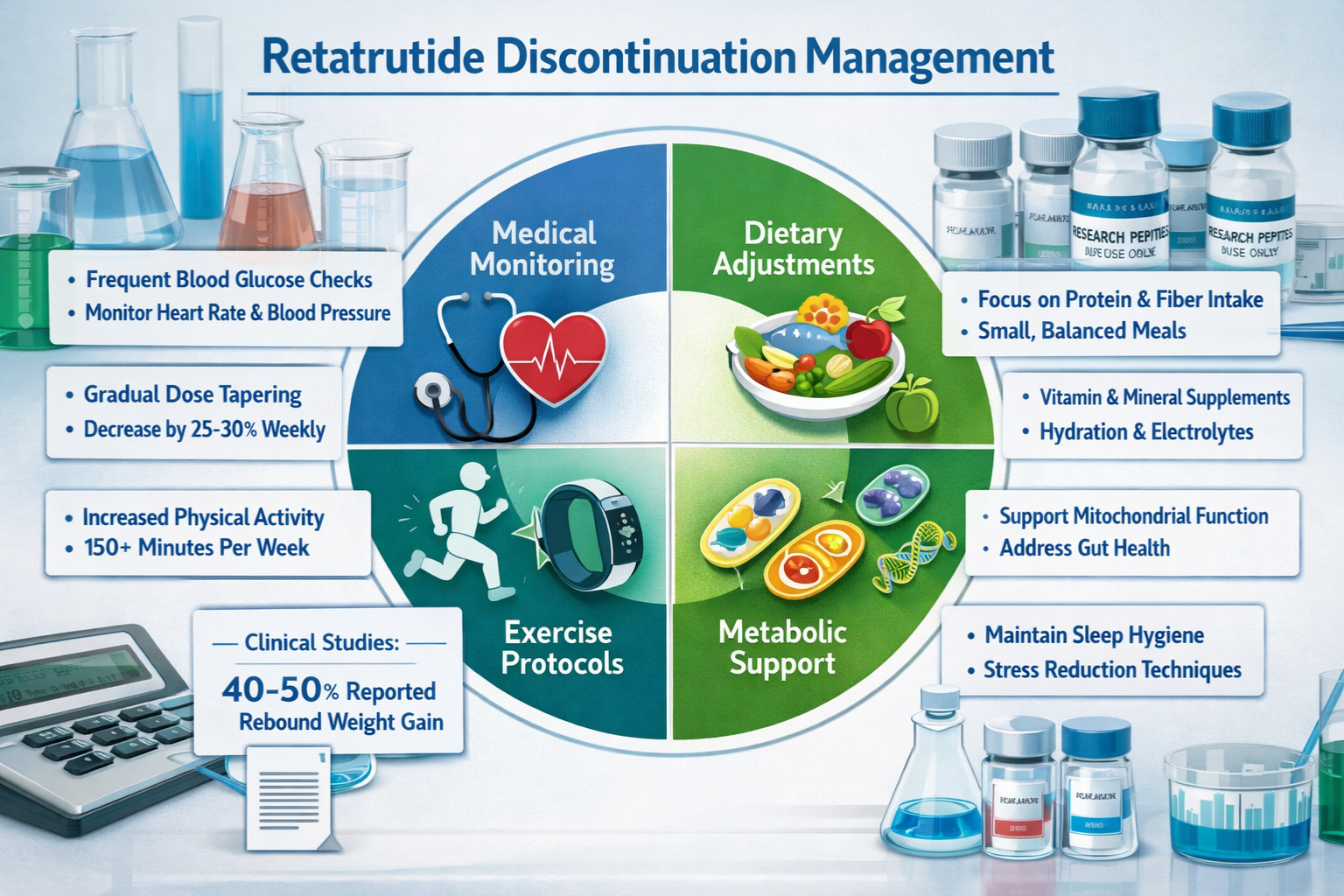
Proper management of the cessation period optimizes research outcomes and ensures the scientific validity of discontinuation studies examining retatrutide stopping what happens.
Gradual Tapering vs. Abrupt Cessation
Research protocols must decide between gradual dose reduction (tapering) and immediate discontinuation, each approach offering distinct advantages for different research questions.
Abrupt cessation approach:
- ✅ Advantages: Clear temporal relationship, simplified protocol, definitive pharmacokinetic assessment
- ⚠️ Considerations: May produce more pronounced rebound effects, potential for acute metabolic shifts
Gradual tapering approach:
- ✅ Advantages: May reduce rebound phenomena, mimics potential clinical applications, smoother metabolic transition
- ⚠️ Considerations: Extended protocol duration, more complex pharmacokinetic interpretation, variable tapering schedules
Supportive Interventions During Cessation
Research protocols may incorporate supportive measures to stabilize metabolic parameters during the discontinuation period, depending on study objectives.
Potential supportive strategies:
- Dietary standardization: Controlled nutrition to minimize confounding metabolic variables
- Activity protocols: Structured exercise regimens to support metabolic stability
- Monitoring frequency: Increased assessment frequency during critical transition periods
- Environmental controls: Maintained housing conditions to reduce external stressors
Washout Period Considerations
For studies involving sequential interventions or crossover designs, adequate washout periods prove essential for eliminating carryover effects.
Washout period guidelines:
- Minimum duration: 12-16 weeks for complete metabolic recovery
- Verification methods: Confirm return to baseline before subsequent interventions
- Individual variation: Allow flexibility for models showing delayed recovery
- Documentation: Thoroughly record washout period observations
Ethical Research Practices
Even in non-clinical research settings, ethical considerations guide proper discontinuation management.
Ethical framework elements:
- 🔬 Scientific justification: Ensure cessation serves legitimate research objectives
- 📋 Protocol approval: Obtain appropriate institutional review and oversight
- 📊 Data transparency: Report both positive and negative cessation outcomes
- ⚖️ Welfare considerations: Monitor for adverse effects during discontinuation
Research teams committed to high-quality peptide research recognize that proper discontinuation management enhances scientific rigor and reproducibility.
Comparing Retatrutide Cessation to Other Peptide Discontinuation
Understanding how retatrutide stopping what happens compares to cessation of other metabolic peptides provides valuable context for researchers and highlights the compound’s unique characteristics.
Single-Agonist GLP-1 Compounds
Traditional GLP-1 receptor agonists show different cessation profiles compared to the triple-agonist retatrutide.
Comparative cessation characteristics:
| Feature | GLP-1 Agonists | Retatrutide |
|---|---|---|
| Elimination half-life | 1-7 days (variable) | 5-7 days |
| Receptor systems affected | Single (GLP-1) | Triple (GLP-1, GIP, glucagon) |
| Metabolic rebound magnitude | Moderate | Potentially greater |
| Recovery timeline | 4-8 weeks | 8-16 weeks |
| Persistent adaptations | Limited | More substantial |
Dual-Agonist Peptides
Dual-agonist compounds (such as tirzepatide, targeting GLP-1 and GIP) represent an intermediate comparison point between single-agonist and triple-agonist peptides.
Key differences in cessation:
- Receptor normalization: Dual-agonists show intermediate recovery timelines
- Metabolic effects: Less pronounced multi-system rebound compared to triple-agonists
- Duration of effects: Moderate persistence of metabolic adaptations
- Research implications: Simpler mechanistic interpretation than triple-agonists
Researchers comparing different peptide classes can explore comprehensive peptide options to design comparative cessation studies.
Growth Hormone Secretagogues
Peptides affecting growth hormone pathways (such as ipamorelin or CJC-1295) demonstrate distinctly different cessation profiles due to their alternative mechanisms of action.
Contrasting cessation patterns:
- 🧬 Mechanism: Indirect metabolic effects via GH/IGF-1 axis vs. direct receptor activation
- ⏱️ Timeline: Variable recovery depending on downstream adaptations
- 📊 Metabolic markers: Different parameter changes during cessation
- 🔬 Research focus: Distinct monitoring requirements and assessment endpoints
Metabolic Support Peptides
Compounds like AOD-9604 or MOTS-C show yet another cessation pattern, reflecting their specific metabolic mechanisms.
Unique cessation characteristics:
- Rapid clearance: Often shorter half-lives than retatrutide
- Minimal rebound: Less pronounced metabolic rebound phenomena
- Specific effects: Targeted metabolic pathway normalization
- Recovery speed: Generally faster return to baseline parameters
Understanding these comparative contexts helps researchers design appropriate protocols when examining retatrutide stopping what happens in their specific experimental systems.
Research Applications and Study Design Considerations
The knowledge gained from understanding retatrutide stopping what happens informs multiple research applications and experimental design decisions.
Crossover Study Designs
Crossover protocols, where the same research models receive multiple treatments in sequence, require careful attention to retatrutide’s cessation characteristics.
Critical design elements:
- Washout duration: Minimum 12-16 weeks between retatrutide exposure and subsequent interventions
- Baseline re-establishment: Verify complete metabolic recovery before crossover
- Carryover analysis: Statistical methods to detect and account for persistent effects
- Sequence randomization: Balance treatment order to minimize systematic bias
Dose-Response Cessation Studies
Investigating how different retatrutide doses influence cessation outcomes provides valuable mechanistic insights.
Research questions to address:
- 📊 Does higher dosing produce more pronounced or prolonged cessation effects?
- ⏱️ How does dose intensity affect recovery timeline to baseline?
- 🔬 Are certain metabolic parameters more dose-sensitive during cessation?
- 📈 What is the relationship between cumulative exposure and persistent adaptations?
Duration-Dependent Effect Studies
Examining how treatment length influences cessation outcomes illuminates the temporal dynamics of metabolic adaptation.
Experimental approach:
| Treatment Duration | Primary Research Focus | Expected Cessation Pattern |
|---|---|---|
| Short-term (4-8 weeks) | Acute pharmacological effects | Rapid reversal, minimal persistence |
| Medium-term (3-6 months) | Metabolic adaptation onset | Moderate persistence, intermediate recovery |
| Long-term (6+ months) | Durable metabolic remodeling | Substantial persistence, extended recovery |
Mechanistic Cessation Research
Advanced research protocols may focus specifically on the mechanisms underlying cessation-related changes.
Mechanistic research approaches:
- Receptor dynamics: Track GLP-1, GIP, and glucagon receptor expression and sensitivity throughout cessation
- Signaling pathways: Examine downstream signaling cascade normalization
- Metabolic flux: Assess changes in nutrient processing and energy partitioning
- Transcriptional regulation: Profile gene expression changes during recovery
- Epigenetic modifications: Investigate potential lasting epigenetic adaptations
Researchers designing comprehensive mechanistic studies benefit from high-purity research peptides that ensure experimental consistency across complex protocols.
Combination Therapy Discontinuation
Research exploring retatrutide in combination with other interventions must consider complex cessation dynamics when multiple treatments are discontinued.
Combination cessation considerations:
- 🔄 Sequential discontinuation: Stagger cessation of different interventions to isolate effects
- ⚡ Simultaneous cessation: Discontinue all treatments together to assess combined withdrawal
- 📊 Interaction effects: Evaluate how combined treatments influence each other’s cessation profiles
�
� Strategic planning: Design protocols that answer specific mechanistic questions
Future Research Directions in Retatrutide Cessation
As the research community continues investigating retatrutide stopping what happens, several promising avenues warrant further exploration.
Personalized Cessation Prediction Models
Developing predictive models that forecast individual cessation outcomes based on baseline characteristics and treatment parameters represents a valuable research frontier.
Potential model inputs:
- Baseline metabolic phenotype
- Genetic polymorphisms affecting receptor function
- Treatment duration and dosing history
- Concurrent interventions and environmental factors
- Early cessation response markers
Biomarkers of Recovery Completeness
Identifying specific biomarkers that reliably indicate complete metabolic recovery would enhance research protocol efficiency.
Candidate biomarkers include:
- 🧬 Receptor expression levels in relevant tissues
- 📊 Specific metabolite ratios reflecting pathway normalization
- ⚡ Functional tests of incretin response
- 🔬 Circulating hormonal profiles
Strategies to Preserve Beneficial Adaptations
Research exploring interventions that maintain retatrutide’s beneficial metabolic effects following cessation could yield valuable insights.
Potential preservation strategies:
- Dietary interventions: Specific nutritional protocols to support metabolic stability
- Exercise regimens: Structured activity programs to maintain adaptations
- Adjunct peptides: Complementary compounds that support metabolic health
- Gradual tapering protocols: Optimized dose reduction schedules
Long-Term Follow-Up Studies
Extended observation periods tracking metabolic parameters months to years post-cessation would illuminate the true durability of retatrutide-induced adaptations.
Long-term research priorities:
- 📈 Multi-year metabolic tracking: Assess whether any adaptations persist beyond 6-12 months
- 🔄 Repeat exposure studies: Examine how prior retatrutide treatment affects subsequent response
- 📊 Cumulative effect analysis: Evaluate whether multiple treatment cycles produce additive adaptations
�
� Optimal re-treatment timing: Determine ideal intervals between treatment courses
Comparative Triple-Agonist Research
As additional triple-agonist peptides enter research pipelines, comparative cessation studies will provide valuable insights into class effects versus compound-specific characteristics.
Comparative research questions:
- How do different triple-agonist structures affect cessation profiles?
- Are cessation characteristics primarily determined by receptor selectivity or pharmacokinetics?
- Do structural modifications influence the durability of metabolic adaptations?
- What design features optimize the balance between efficacy and cessation manageability?
Researchers pursuing these advanced investigations can access specialized research peptides to support comprehensive comparative studies.
Practical Recommendations for Researchers
Based on current understanding of retatrutide stopping what happens, several practical recommendations guide optimal research practice.
Protocol Design Recommendations
✅ Plan adequate washout periods: Allow minimum 12-16 weeks between retatrutide exposure and subsequent interventions in crossover designs
✅ Establish comprehensive baseline: Thoroughly characterize metabolic parameters before treatment initiation to enable accurate cessation comparisons
✅ Implement systematic monitoring: Schedule regular assessments throughout the cessation period to capture dynamic changes
✅ Control confounding variables: Standardize diet, activity, and environmental conditions during cessation observation
✅ Document thoroughly: Maintain detailed records of all cessation-related observations, including unexpected findings
Analytical Approach Recommendations
📊 Pre-specify statistical methods: Determine analytical approaches for cessation data before study initiation
📊 Account for individual variation: Use appropriate statistical models that accommodate inter-subject variability in recovery timelines
📊 Analyze temporal patterns: Employ longitudinal analytical methods to characterize time-dependent changes
📊 Report complete data: Present both positive and null findings regarding cessation effects
📊 Consider mechanistic analyses: Incorporate exploratory analyses to generate hypotheses about cessation mechanisms
Quality Assurance Recommendations
🔬 Source high-purity peptides: Utilize research-grade retatrutide from reputable suppliers to ensure experimental consistency
🔬 Verify peptide identity: Confirm compound identity and purity through appropriate analytical methods
🔬 Maintain proper storage: Follow recommended storage conditions throughout the study period
🔬 Document handling procedures: Record reconstitution, storage, and administration details
🔬 Implement quality controls: Include appropriate positive and negative controls in experimental designs
Collaboration and Knowledge Sharing
🤝 Engage with research community: Share cessation findings through appropriate scientific channels
🤝 Participate in collaborative studies: Join multi-site research efforts to increase statistical power
🤝 Contribute to databases: Provide data to centralized repositories studying peptide cessation
🤝 Attend scientific meetings: Present findings and learn from other researchers’ experiences
🤝 Publish comprehensively: Report cessation data even in studies primarily focused on treatment effects
Conclusion
Understanding retatrutide stopping what happens represents a critical component of comprehensive research with this innovative triple-agonist peptide. The cessation period reveals important insights into the compound’s mechanism of action, duration of effect, and the nature of metabolic adaptations induced during treatment.
Key findings from current research indicate that retatrutide cessation follows a predictable timeline, with pharmacokinetic elimination occurring over 5-6 weeks (approximately five half-lives) and complete metabolic recovery typically achieved within 12-16 weeks post-discontinuation. However, significant individual variation exists, influenced by treatment duration, dosing protocols, baseline metabolic characteristics, and concurrent interventions.
The multi-receptor mechanism of retatrutide—simultaneously targeting GLP-1, GIP, and glucagon pathways—produces more complex cessation dynamics compared to single-agonist compounds. Researchers must account for the normalization of three distinct receptor systems, each with potentially different recovery timelines, when designing discontinuation studies and interpreting cessation data.
Proper management of the retatrutide cessation period requires systematic monitoring of metabolic parameters, careful control of confounding variables, and adequate washout periods in sequential treatment designs. Advanced research applications continue to illuminate the mechanisms underlying cessation-related changes and identify strategies for preserving beneficial metabolic adaptations following treatment discontinuation.
Next Steps for Researchers
For laboratories planning retatrutide cessation studies or seeking to understand discontinuation dynamics in their specific research contexts:
- Review current protocols: Ensure adequate cessation monitoring and washout periods are incorporated
- Source quality peptides: Obtain high-purity research-grade retatrutide from established suppliers
- Consult expertise: Contact specialized peptide providers for guidance on protocol optimization
- Plan comprehensively: Design studies that capture both treatment effects and cessation dynamics
- Share findings: Contribute to the growing knowledge base regarding retatrutide discontinuation
The field of peptide research continues advancing rapidly, with retatrutide representing an exciting frontier in metabolic investigation. By thoroughly understanding what happens when retatrutide stopping occurs, researchers can design more robust experimental protocols, interpret findings more accurately, and contribute valuable knowledge to the scientific community.
As research progresses into 2026 and beyond, the insights gained from systematic cessation studies will inform optimal research practices, enhance mechanistic understanding, and potentially guide future applications of this remarkable triple-agonist compound.
