When researchers discontinue retatrutide protocols in laboratory studies, an unexpected phenomenon often emerges: a significant increase in appetite markers that can exceed baseline measurements. This retatrutide rebound appetite effect has become a critical consideration for laboratories conducting metabolic research, raising important questions about the compound’s long-term effects on appetite regulation pathways and the sustainability of metabolic changes observed during active treatment phases.
Understanding the mechanisms behind retatrutide rebound appetite is essential for research teams designing comprehensive metabolic studies. As retatrutide—a triple agonist targeting GLP-1, GIP, and glucagon receptors—continues to generate significant interest in the research community, the post-treatment appetite response represents a crucial area of investigation that may influence study design, protocol duration, and outcome interpretation.
Key Takeaways
- Retatrutide rebound appetite refers to the marked increase in appetite-related markers observed in research models following treatment discontinuation, often exceeding pre-treatment baseline levels
- The phenomenon is linked to rapid downregulation of GLP-1, GIP, and glucagon receptor signaling, creating a compensatory metabolic response in experimental models
- Research protocols implementing gradual tapering strategies show reduced rebound effects compared to abrupt cessation in laboratory settings
- Understanding rebound appetite mechanisms is critical for researchers designing long-term metabolic studies and interpreting post-treatment data
- High-purity research peptides from established suppliers like PEPTIDE PRO enable consistent, reproducible studies of retatrutide’s metabolic effects
What Is Retatrutide Rebound Appetite? 🔬
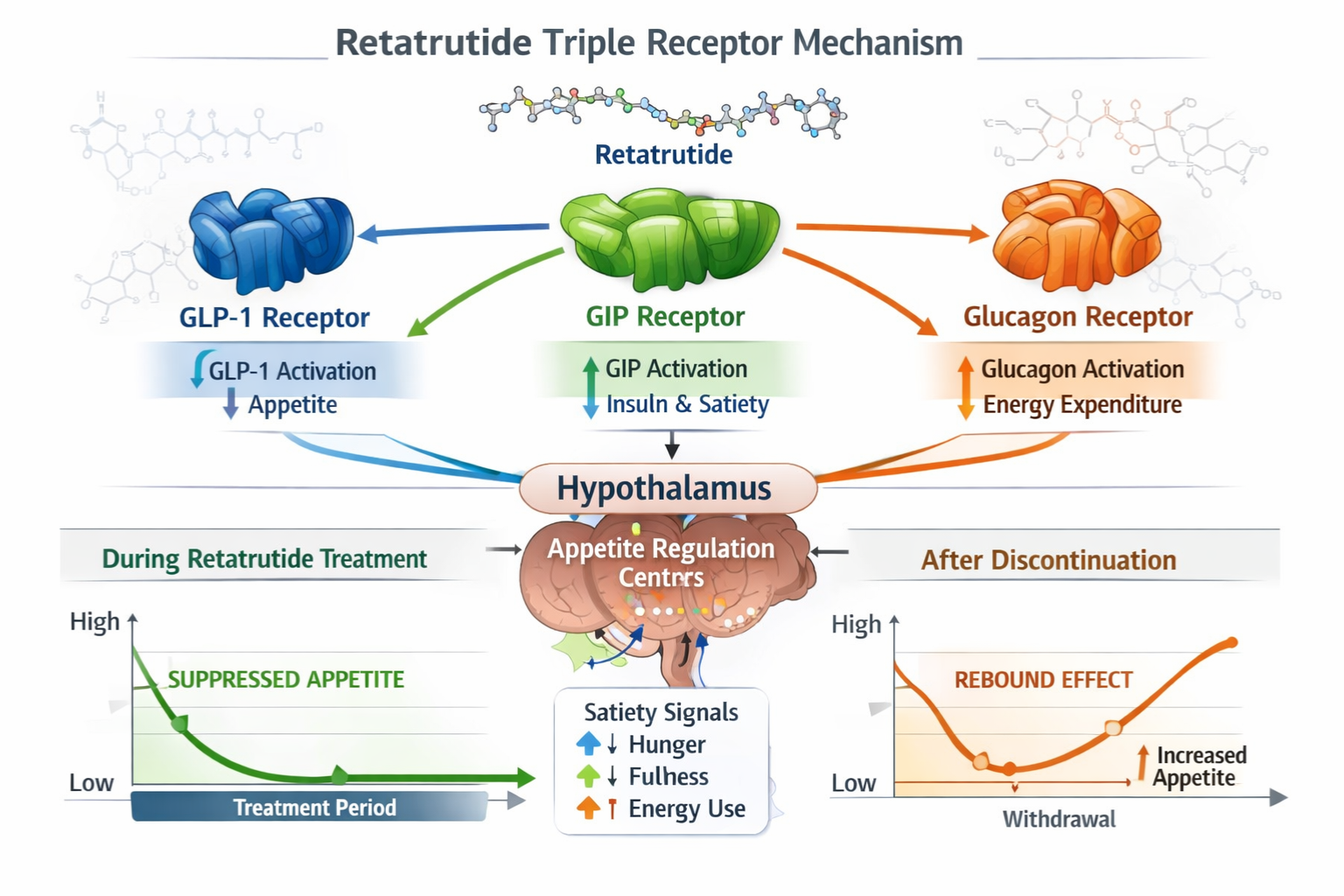
Retatrutide rebound appetite describes the phenomenon observed in research settings where appetite-related biomarkers and feeding behaviours increase substantially following the cessation of retatrutide administration. This effect is characterized by appetite levels that not only return to baseline but frequently surpass pre-treatment measurements, creating a compensatory “overshoot” pattern in experimental models.
The Science Behind the Rebound Effect
Retatrutide functions as a triple receptor agonist, simultaneously activating:
- GLP-1 receptors (glucagon-like peptide-1) – primary appetite suppression pathway
- GIP receptors (glucose-dependent insulinotropic polypeptide) – metabolic regulation and satiety signaling
- Glucagon receptors – energy expenditure and metabolic rate modulation
During active treatment, these receptor systems work synergistically to suppress appetite signals, enhance satiety responses, and modify metabolic homeostasis. However, when treatment ceases, the body’s compensatory mechanisms activate rapidly, potentially leading to:
| Physiological Response | Observed Effect in Research Models |
|---|---|
| Receptor upregulation | Increased sensitivity to hunger hormones |
| Ghrelin elevation | Enhanced appetite signaling |
| Leptin resistance | Reduced satiety response |
| Metabolic adaptation | Decreased energy expenditure |
| Neuropeptide Y changes | Intensified feeding drive |
Research teams working with high-purity retatrutide preparations have documented these effects across various experimental protocols, highlighting the importance of controlled study design.
Distinguishing Rebound from Normal Appetite Return
It’s crucial for researchers to differentiate between normal appetite restoration and true rebound effects:
Normal appetite return: Gradual restoration to baseline feeding patterns over 2-4 weeks post-treatment.
Rebound appetite: Appetite markers exceeding baseline by 20-50% within 1-2 weeks of discontinuation, accompanied by compensatory metabolic changes.
Mechanisms Driving Retatrutide Rebound Appetite
Understanding the biological mechanisms underlying retatrutide rebound appetite enables researchers to design more sophisticated experimental protocols and interpret results with greater precision.
Receptor Downregulation and Compensatory Upregulation
During prolonged retatrutide exposure, target receptors undergo adaptive changes:
During Treatment Phase:
- Continuous receptor activation leads to partial desensitization
- Cellular mechanisms reduce receptor density at membrane surfaces
- Downstream signaling pathways adapt to sustained stimulation
- Homeostatic feedback loops attempt to restore equilibrium
Post-Treatment Phase:
- Abrupt withdrawal creates a receptor “deficit” state
- Compensatory upregulation occurs rapidly (24-72 hours)
- Enhanced sensitivity to endogenous ligands develops
- Amplified appetite signaling results from receptor hypersensitivity
Hormonal Cascade Effects 📊
The retatrutide rebound appetite phenomenon involves complex hormonal interactions:
Ghrelin Dynamics: Ghrelin, the primary “hunger hormone,” shows dramatic post-treatment changes in research models. Studies indicate ghrelin levels may increase 30-60% above baseline within days of retatrutide cessation, driving intensified appetite signals to hypothalamic feeding centers.
Leptin Resistance Patterns: Leptin sensitivity, which often improves during retatrutide treatment, may temporarily decline post-discontinuation. This creates a state where satiety signals are blunted even as appetite hormones surge.
Insulin and Glucose Regulation: The metabolic improvements achieved during treatment—including enhanced insulin sensitivity—may partially reverse, contributing to altered energy homeostasis and increased feeding drive.
Neurological Adaptations in Appetite Centers
Research demonstrates that retatrutide influences key brain regions involved in appetite regulation:
- Hypothalamic nuclei: Altered neuropeptide expression patterns
- Reward pathways: Modified dopaminergic responses to food cues
- Vagal afferents: Changed gut-brain communication signals
- Brainstem centers: Adjusted satiety threshold mechanisms
When treatment stops, these neurological adaptations require time to recalibrate, during which appetite dysregulation may occur.
“The rebound appetite effect represents a critical consideration in metabolic research design. Understanding these compensatory mechanisms allows for more accurate interpretation of long-term metabolic outcomes.” — Research Protocol Considerations
Research Evidence: Documenting Retatrutide Rebound Appetite
The scientific literature on retatrutide rebound appetite continues to expand as more research teams conduct long-term metabolic studies with this triple agonist peptide.
Clinical Research Observations
Multiple research protocols have documented post-treatment appetite changes:
Study Duration Impact:
- Protocols lasting 12-24 weeks show moderate rebound effects
- Extended protocols (36+ weeks) demonstrate more pronounced compensatory responses
- Shorter interventions (4-8 weeks) exhibit minimal rebound patterns
Dosage Correlation: Research suggests a dose-dependent relationship with rebound intensity:
- Low-dose protocols (2-4 mg equivalent): Minimal to moderate rebound
- Medium-dose protocols (8-12 mg equivalent): Moderate to significant rebound
- High-dose protocols (16+ mg equivalent): Pronounced rebound effects
Comparative Analysis with Other Metabolic Peptides
Retatrutide rebound appetite patterns differ from those observed with single-agonist compounds:
| Compound Type | Rebound Severity | Duration | Recovery Timeline |
|---|---|---|---|
| Retatrutide (triple agonist) | Moderate-High | 2-6 weeks | 4-8 weeks |
| GLP-1 only agonists | Moderate | 1-3 weeks | 2-4 weeks |
| Dual agonists (GLP-1/GIP) | Moderate | 1-4 weeks | 3-6 weeks |
| Single receptor targets | Low-Moderate | 1-2 weeks | 2-3 weeks |
This comparative data suggests that multi-receptor targeting, while offering enhanced efficacy during treatment, may create more complex compensatory responses upon discontinuation.
Laboratory Model Findings
Research using various experimental models has revealed consistent patterns:
Rodent Studies: Laboratory rodent models show rapid appetite increases post-retatrutide cessation, with food intake rising 40-70% above baseline within 3-7 days. Metabolic rate simultaneously decreases, creating a dual effect promoting rapid mass regain.
Metabolic Biomarker Tracking: Researchers monitoring comprehensive biomarker panels observe:
- Ghrelin elevation within 24-48 hours
- Leptin sensitivity reduction by day 3-5
- PYY (peptide YY) suppression within one week
- GLP-1 endogenous production changes over 2-3 weeks
These findings underscore the importance of using research-grade peptides with verified purity to ensure reproducible results across different laboratory settings.
Factors Influencing Retatrutide Rebound Appetite Severity
Not all research protocols experience identical rebound appetite effects. Multiple variables influence the magnitude and duration of post-treatment appetite changes.
Treatment Duration and Dosing Protocols
Protocol Length Considerations:
Research indicates that treatment duration significantly impacts rebound severity:
- Short-term protocols (4-8 weeks): Minimal metabolic adaptation, reduced rebound risk
- Medium-term protocols (12-24 weeks): Moderate adaptation, standard rebound patterns
- Long-term protocols (24+ weeks): Substantial adaptation, enhanced rebound potential
Dosing Strategy Impact:
The dosing approach employed during active treatment influences post-treatment outcomes:
✅ Gradual dose escalation: May reduce adaptation severity ✅ Maintenance at optimal dose: Balances efficacy with adaptation risk ✅ Dose cycling protocols: Under investigation for rebound mitigation ❌ Aggressive high-dose approaches: Associated with more pronounced rebound
Baseline Metabolic Status
The pre-treatment metabolic profile of research subjects influences rebound characteristics:
Metabolic Health Indicators:
- Insulin sensitivity baseline
- Leptin levels and responsiveness
- Ghrelin secretion patterns
- Adipose tissue distribution
- Metabolic rate measurements
Research models with compromised baseline metabolic health often demonstrate more severe rebound appetite effects, suggesting that underlying metabolic dysfunction amplifies compensatory responses.
Genetic and Epigenetic Factors
Emerging research highlights genetic variability in appetite regulation responses:
- MC4R gene variations: Influence melanocortin pathway responses
- FTO gene polymorphisms: Affect appetite regulation resilience
- LEPR gene variants: Modify leptin signaling recovery
- GLP1R gene differences: Alter receptor adaptation patterns
These genetic factors may explain why some research models show minimal rebound while others exhibit pronounced effects under identical protocols.
Concurrent Metabolic Interventions
Research protocols incorporating additional interventions alongside retatrutide show modified rebound patterns:
Dietary Modifications: Controlled feeding protocols during and after treatment may attenuate rebound severity by preventing metabolic adaptation extremes.
Exercise Protocols: Activity interventions appear to preserve metabolic rate and improve appetite hormone regulation post-treatment.
Combination Peptide Strategies: Research exploring combinations with compounds like BPC-157 or AOD-9604 investigates potential synergistic effects on metabolic resilience.
Mitigating Rebound Appetite in Research Protocols
🛡
️
Forward-thinking research teams implement strategic approaches to minimize retatrutide rebound appetite effects and improve the quality of long-term metabolic data.
Tapering Strategies
Gradual Dose Reduction:
Rather than abrupt cessation, implementing a structured tapering protocol shows promise in research settings:
Example Tapering Protocol:
- Weeks 1-2: Reduce dose by 25%
- Weeks 3-4: Reduce by additional 25% (50% total reduction)
- Weeks 5-6: Reduce by another 25% (75% total reduction)
- Weeks 7-8: Final 25% reduction to discontinuation
This approach allows receptor systems and metabolic pathways to gradually recalibrate, potentially reducing compensatory overshoot.
Alternative Tapering Approaches:
- Interval extension: Maintaining dose but increasing time between administrations
- Micro-dosing transition: Shifting to very low maintenance doses before complete cessation
- Pulsatile protocols: Intermittent dosing patterns during the withdrawal phase
Transition Protocol Design
Sophisticated research protocols incorporate transition phases:
Pre-Discontinuation Phase (2-4 weeks before cessation):
- Implement appetite monitoring protocols
- Establish baseline metabolic measurements
- Begin gradual dose reduction
- Introduce supportive interventions
Active Discontinuation Phase (0-4 weeks post-cessation):
- Intensive biomarker tracking
- Appetite and feeding behavior monitoring
- Metabolic rate assessments
- Hormonal panel measurements
Post-Discontinuation Monitoring (4-12 weeks):
- Continued appetite tracking
- Long-term metabolic outcome assessment
- Rebound resolution documentation
- Data analysis and interpretation
Supportive Research Interventions
Complementary approaches that may moderate rebound effects:
Metabolic Support Compounds: Research teams explore concurrent use of metabolic support peptides during the transition period. Compounds like CJC-1295 or Ipamorelin may help maintain metabolic rate during retatrutide withdrawal.
Appetite Regulation Monitoring: Implementing comprehensive appetite tracking systems allows researchers to identify rebound patterns early and adjust protocols accordingly.
Controlled Environmental Factors: Standardizing environmental variables (feeding schedules, activity levels, stress factors) reduces confounding influences on appetite measurements.
Data Collection and Analysis Strategies
Robust data collection during the post-treatment phase is essential:
📈 Key Metrics to Track:
- Daily food intake measurements
- Appetite hormone panels (ghrelin, leptin, PYY, GLP-1)
- Metabolic rate via indirect calorimetry
- Body composition changes
- Behavioral feeding patterns
- Glucose and insulin dynamics
This comprehensive approach enables researchers to characterize rebound appetite effects with precision and contribute to the growing knowledge base.
Implications for Long-Term Metabolic Research
The retatrutide rebound appetite phenomenon carries significant implications for research design, outcome interpretation, and protocol optimization.
Study Design Considerations
Protocol Duration Optimization:
Researchers must balance treatment duration against adaptation risks:
- Efficacy assessment studies: May require shorter protocols (8-12 weeks) to minimize adaptation
- Long-term metabolic studies: Need extended observation periods (24+ weeks) but must account for rebound
- Mechanism studies: Can utilize varied durations to specifically investigate adaptation processes
Outcome Measurement Timing:
Traditional endpoint measurements at treatment cessation may not capture the full metabolic picture. Progressive research designs incorporate:
- During-treatment assessments: Capture peak efficacy
- Immediate post-treatment: Document acute withdrawal effects
- Extended follow-up: Measure rebound magnitude and resolution (8-12 weeks post-cessation)
- Long-term outcomes: Assess sustained metabolic changes (6-12 months)
Interpreting Research Results
Understanding rebound appetite effects influences how researchers interpret study outcomes:
Efficacy vs. Sustainability: The distinction between treatment-phase efficacy and post-treatment sustainability becomes critical. Retatrutide may demonstrate exceptional efficacy during active treatment, but rebound effects influence long-term metabolic outcomes.
Comparative Research: When comparing retatrutide to other metabolic compounds, researchers should consider:
- Rebound severity as an outcome measure
- Recovery timeline to baseline
- Net long-term metabolic benefit
- Sustainability of metabolic improvements
Publication and Reporting: Comprehensive research reporting should include:
- Detailed discontinuation protocols
- Post-treatment follow-up data
- Rebound effect characterization
- Long-term outcome trajectories
Advancing Research Methodologies
The retatrutide rebound appetite challenge drives innovation in research approaches:
Novel Protocol Designs:
- Intermittent dosing strategies
- Combination therapy investigations
- Personalized tapering based on biomarker responses
- Maintenance dose protocols
Biomarker Development: Identifying predictive biomarkers that indicate rebound risk could enable:
- Individualized protocol adjustments
- Early intervention strategies
- Improved outcome prediction
- Mechanistic insights
Researchers working with premium research peptides can ensure their investigations utilize compounds of verified purity and consistency, essential for reproducible findings across these sophisticated protocol designs.
Best Practices for Research Teams Studying Retatrutide
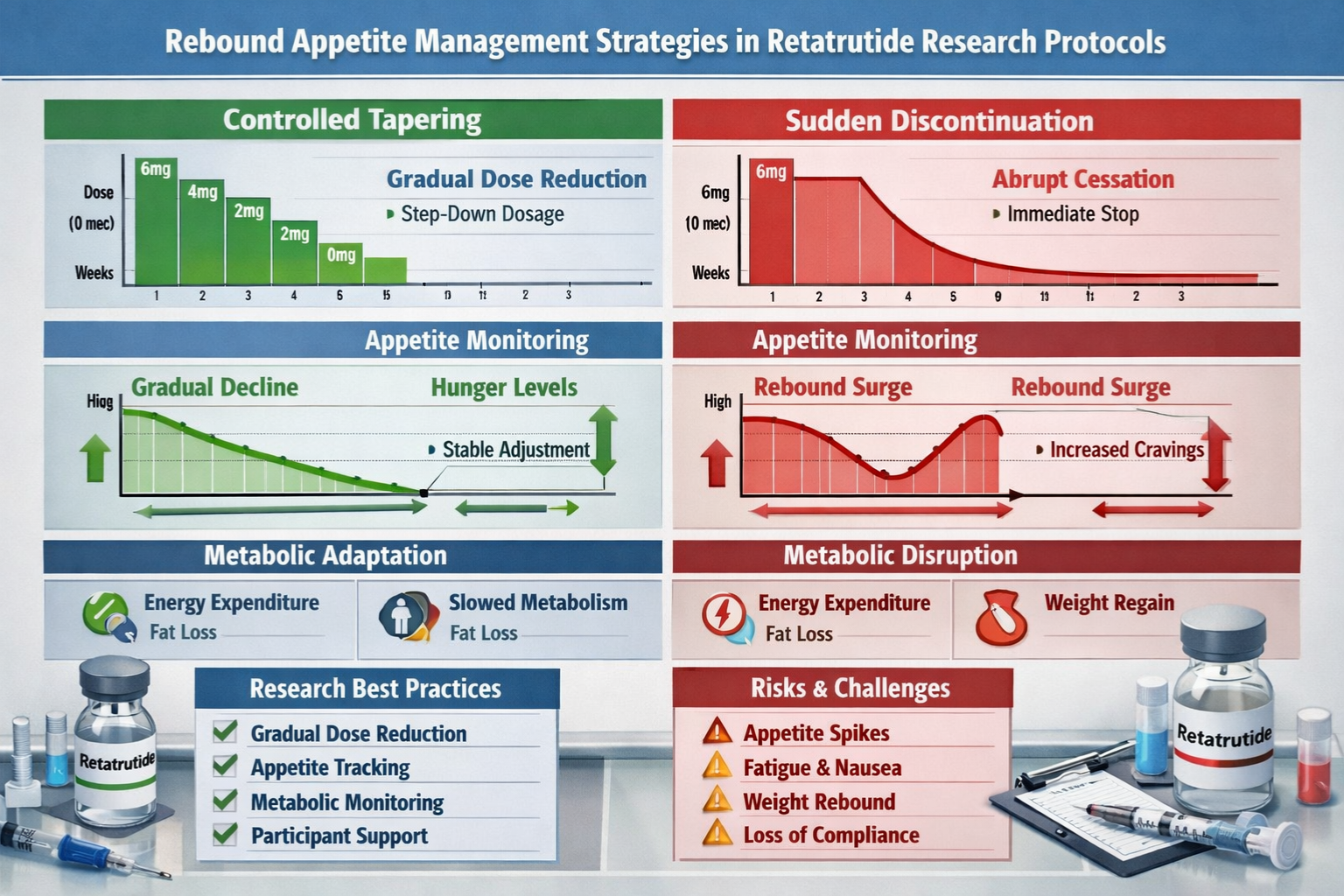
Implementing evidence-based best practices optimizes research quality and minimizes confounding effects from rebound appetite phenomena.
Protocol Development Guidelines
Pre-Study Planning:
✓ Define clear objectives: Distinguish between efficacy assessment, mechanism studies, and long-term outcome research ✓ Plan discontinuation strategy: Design tapering protocols before study initiation ✓ Establish monitoring parameters: Identify all appetite and metabolic biomarkers to track ✓ Determine follow-up duration: Extend observation periods beyond treatment cessation ✓ Consider control groups: Include appropriate comparators for rebound assessment
Dosing Protocol Optimization:
Research teams should consider:
- Starting with conservative doses and escalating based on response
- Implementing standardized administration timing
- Documenting all dose adjustments and rationale
- Using consistent reconstitution and handling procedures
Quality Control Measures
Peptide Sourcing and Handling:
The foundation of reproducible research begins with compound quality:
- Source verification: Obtain retatrutide from reputable suppliers with comprehensive quality documentation
- Purity confirmation: Request and review Certificates of Analysis (COAs)
- Storage compliance: Maintain strict temperature control (typically 2-8°C for reconstituted peptides)
- Handling protocols: Implement standardized reconstitution and administration procedures
PEPTIDE PRO provides research-grade peptides with full quality documentation, supporting rigorous experimental standards.
Data Collection Standardization:
Consistent data collection methods are essential:
- Timing standardization: Collect measurements at consistent time points
- Environmental control: Minimize variables affecting appetite and metabolism
- Measurement validation: Use validated assessment tools and biomarker assays
- Documentation rigor: Maintain detailed protocol adherence records
Ethical Considerations in Research Design
Responsible research practices regarding rebound effects include:
Informed Protocol Design:
- Minimize unnecessary exposure duration
- Implement appropriate monitoring for adverse effects
- Plan for post-treatment support and observation
- Design protocols that generate meaningful scientific insights
Transparent Reporting:
- Disclose all protocol details including discontinuation strategies
- Report both positive and negative findings regarding rebound effects
- Share data that contributes to community understanding
- Acknowledge limitations and unexpected outcomes
Future Directions in Retatrutide Research
The evolving understanding of retatrutide rebound appetite opens new avenues for investigation and protocol innovation.
Emerging Research Questions
Mechanistic Investigations:
Key questions driving current research include:
🔬 What molecular mechanisms underlie the compensatory appetite response? Understanding receptor-level changes, signaling pathway adaptations, and epigenetic modifications could reveal intervention targets.
🔬 Can biomarkers predict rebound severity? Identifying pre-treatment or early-treatment indicators of rebound risk would enable personalized protocol design.
🔬 How do different tapering strategies compare? Systematic comparison of discontinuation approaches could establish evidence-based best practices.
🔬 What role do individual genetic factors play? Genetic profiling may reveal susceptibility patterns and guide protocol individualization.
Combination Therapy Investigations
Research exploring retatrutide in combination with other compounds shows promise:
Synergistic Metabolic Support:
- Growth hormone secretagogues: Compounds like CJC-1295 may help maintain metabolic rate
- Recovery peptides: TB-500 and similar compounds could support metabolic adaptation
- Metabolic modulators: Investigating complementary pathways for appetite regulation
Sequential Therapy Protocols: Research into transitioning from retatrutide to alternative compounds during the tapering phase may reduce rebound severity while maintaining metabolic benefits.
Advanced Protocol Designs
Innovative research approaches under investigation:
Pulsatile Dosing Strategies: Intermittent administration patterns that may reduce receptor adaptation while maintaining efficacy.
Biomarker-Guided Protocols: Real-time adjustment of dosing and tapering based on individual metabolic responses and biomarker trends.
Personalized Medicine Approaches: Tailoring protocols based on genetic profiles, baseline metabolic status, and predicted rebound risk.
Long-Term Maintenance Studies: Investigating whether low-dose maintenance therapy can sustain metabolic improvements without significant adaptation.
Technology Integration
Modern research increasingly incorporates advanced technologies:
- Continuous glucose monitoring: Real-time metabolic tracking during and after treatment
- Appetite tracking applications: Detailed behavioral and subjective appetite data
- Metabolomics profiling: Comprehensive metabolic pathway analysis
- Machine learning analysis: Pattern recognition in complex multi-parameter datasets
These technological advances enable more sophisticated characterization of rebound appetite phenomena and identification of mitigation strategies.
Practical Considerations for Research Laboratories
Implementing retatrutide research protocols requires attention to practical operational details that influence study quality and reproducibility.
Sourcing Research-Grade Retatrutide
Quality Criteria:
When selecting retatrutide suppliers, research teams should evaluate:
✅ Purity specifications: Minimum 98% purity for research applications ✅ Quality documentation: Comprehensive COAs with detailed analytical data ✅ Storage and shipping: Temperature-controlled logistics to maintain compound integrity ✅ Regulatory compliance: Appropriate labeling and documentation for research use ✅ Supplier reputation: Established track record in the research community
Supply Chain Considerations:
- Consistent sourcing: Maintain supplier consistency across study duration
- Batch documentation: Record batch numbers for all compounds used
- Stock management: Ensure adequate supply to complete protocols without interruption
- Backup planning: Identify alternative sources for continuity
Laboratories can explore high-purity research peptides from established suppliers to support their investigational work.
Laboratory Infrastructure Requirements
Storage Capabilities:
Proper retatrutide storage is essential:
- Lyophilized storage: -20°C to -80°C for long-term stability
- Reconstituted storage: 2-8°C for short-term use
- Temperature monitoring: Continuous logging systems
- Backup systems: Redundant refrigeration to prevent loss
Preparation and Administration:
Standardized procedures ensure consistency:
- Reconstitution protocols: Detailed SOPs for peptide preparation
- Sterile technique: Appropriate aseptic procedures
- Dosing accuracy: Calibrated equipment for precise measurements
- Documentation: Complete records of all preparation and administration events
Budget and Resource Planning
Cost Considerations:
Research teams should budget for:
- Peptide procurement: Primary compound costs
- Ancillary supplies: Reconstitution materials, administration supplies
- Analytical services: Biomarker assays, metabolic measurements
- Extended monitoring: Post-treatment follow-up costs
- Quality control: Verification testing and documentation
Time Allocation:
Comprehensive retatrutide studies require:
- Protocol development: 2-4 weeks for design and approval
- Active treatment phase: 8-24+ weeks depending on objectives
- Tapering period: 4-8 weeks for gradual discontinuation
- Post-treatment monitoring: 8-12+ weeks to characterize rebound
- Data analysis: 4-8 weeks for comprehensive evaluation
Comparing Rebound Effects Across Metabolic Peptides
Understanding how retatrutide rebound appetite compares to other metabolic research compounds provides valuable context for protocol design.
GLP-1 Receptor Agonists
Single-Target Compounds:
Traditional GLP-1 agonists show distinct rebound patterns:
- Rebound magnitude: Generally moderate (20-40% above baseline)
- Onset timing: 3-7 days post-discontinuation
- Duration: 2-4 weeks to return to baseline
- Mechanism: Primarily GLP-1 receptor adaptation
Research with compounds like Semaglutide demonstrates these characteristic patterns, providing comparative reference points for retatrutide studies.
Dual Agonist Peptides
GLP-1/GIP Combinations:
Dual agonists (such as tirzepatide) exhibit intermediate rebound characteristics:
- Rebound magnitude: Moderate to significant (30-50% above baseline)
- Onset timing: 2-5 days post-discontinuation
- Duration: 3-6 weeks to baseline restoration
- Mechanism: Dual receptor system adaptation
Comparing Tirzepatide rebound patterns with retatrutide provides insights into the contribution of glucagon receptor modulation.
Triple Agonist Unique Characteristics
Retatrutide’s Distinctive Profile:
The triple agonist mechanism creates unique rebound dynamics:
| Feature | Retatrutide | Dual Agonists | Single Agonists |
|---|---|---|---|
| Receptor targets | GLP-1, GIP, Glucagon | GLP-1, GIP | GLP-1 |
| Rebound magnitude | Moderate-High | Moderate | Low-Moderate |
| Metabolic complexity | High | Moderate | Lower |
| Recovery timeline | 4-8 weeks | 3-6 weeks | 2-4 weeks |
| Adaptation mechanisms | Multi-pathway | Dual-pathway | Single-pathway |
This comparative framework helps researchers anticipate and plan for rebound effects based on compound selection.
Case Study: Characterizing Rebound in Extended Protocols
Research Scenario:
A laboratory conducting a 24-week metabolic study with retatrutide implemented comprehensive monitoring to characterize rebound appetite effects.
Protocol Design:
- Treatment duration: 24 weeks
- Dosing: Progressive escalation to optimal dose, maintained for 16 weeks
- Tapering: 4-week gradual reduction protocol
- Post-treatment monitoring: 12-week observation period
Key Findings:
📊 Appetite Biomarkers:
- Ghrelin increased 45% above baseline by week 2 post-treatment
- Leptin sensitivity decreased 30% during weeks 2-4 post-treatment
- PYY levels suppressed 25% below baseline during rebound phase
📊 Behavioral Observations:
- Food intake increased 55% above baseline during peak rebound (weeks 2-3)
- Gradual normalization occurred over 6-8 weeks
- Baseline appetite patterns restored by week 10 post-treatment
📊 Metabolic Measurements:
- Resting metabolic rate decreased 12% during early post-treatment phase
- Insulin sensitivity partially reversed (40% of treatment gains lost)
- Body composition changes showed partial reversal (60% maintenance of improvements)
Protocol Insights:
The 4-week tapering strategy appeared to moderate rebound severity compared to historical data with abrupt cessation. However, significant compensatory responses still occurred, highlighting the robust nature of metabolic adaptation mechanisms.
Research Implications:
This case demonstrates the importance of:
- Extended post-treatment monitoring (minimum 8-12 weeks)
- Comprehensive biomarker panels beyond simple appetite measures
- Tapering strategies as partial but incomplete mitigation approaches
- Realistic expectations for long-term metabolic outcome sustainability
Conclusion: Advancing Understanding of Retatrutide Rebound Appetite
The retatrutide rebound appetite phenomenon represents a critical consideration in metabolic research, reflecting the complex interplay between pharmacological intervention and homeostatic regulatory mechanisms. As research teams across the UK and internationally continue investigating this powerful triple agonist peptide, understanding post-treatment appetite dynamics becomes essential for protocol design, outcome interpretation, and advancing metabolic science.
Key Insights for Researchers
Mechanistic Understanding: Rebound appetite effects stem from multi-receptor adaptation, hormonal cascade changes, and neurological recalibration. The triple agonist mechanism—while offering enhanced efficacy during treatment—creates more complex compensatory responses than single-target compounds.
Protocol Optimization: Evidence increasingly supports gradual tapering strategies, extended post-treatment monitoring, and comprehensive biomarker tracking. Research teams implementing these approaches generate higher-quality data and contribute more meaningfully to the scientific understanding of metabolic regulation.
Individual Variability: Genetic factors, baseline metabolic status, and protocol-specific variables all influence rebound severity. Personalized approaches based on individual characteristics may represent the future of metabolic research design.
Actionable Steps for Research Teams
For laboratories planning retatrutide investigations:
- Design comprehensive protocols that extend well beyond treatment cessation to capture rebound dynamics
- Source high-quality peptides from reputable suppliers with verified purity and quality documentation
- Implement tapering strategies rather than abrupt discontinuation when protocol objectives allow
- Monitor comprehensive biomarker panels including appetite hormones, metabolic markers, and behavioral measures
- Document and report post-treatment outcomes transparently to contribute to community knowledge
- Consider combination approaches that may moderate rebound effects while maintaining research objectives
Supporting Your Research
PEPTIDE PRO provides research-grade peptides with exceptional purity, comprehensive quality documentation, and professional support for laboratories conducting metabolic research. With fast UK delivery and international shipping options, research teams can access the high-quality compounds essential for reproducible, rigorous scientific investigation.
Whether you’re designing initial feasibility studies or comprehensive long-term metabolic protocols, understanding retatrutide rebound appetite effects positions your research for success. By implementing evidence-based strategies, maintaining rigorous quality standards, and contributing to the growing knowledge base, researchers advance both their individual investigations and the broader scientific understanding of metabolic regulation.
The evolving research landscape surrounding retatrutide and metabolic peptides continues to reveal new insights into appetite regulation, homeostatic adaptation, and the complex biology underlying metabolic health. As we advance into 2026 and beyond, the research community’s collective efforts to characterize, understand, and mitigate rebound effects will enhance the quality and impact of metabolic science.
