When conducting research with advanced peptides like retatrutide, understanding the retatrutide washout period isn’t just a procedural formality—it’s a critical component that can determine the validity and reliability of your experimental outcomes. As research laboratories across the UK and internationally expand their investigations into triple-agonist peptides, the question of appropriate clearance intervals has become increasingly important for maintaining scientific integrity and ensuring accurate, reproducible results.
The retatrutide washout period represents the time required for this novel peptide compound to be adequately eliminated from biological systems before initiating new experimental protocols or transitioning between research phases. With retatrutide’s unique pharmacokinetic profile and extended half-life, establishing proper washout protocols has become essential for researchers working with this compound in laboratory settings.
Key Takeaways
- The retatrutide washout period typically ranges from 4-12 weeks depending on dosage, frequency, and specific research protocols, with most laboratories implementing a minimum 8-week clearance interval
- Pharmacokinetic properties including retatrutide’s extended half-life of approximately 6-7 days necessitate longer washout periods compared to shorter-acting peptides
- Proper washout documentation is essential for research integrity, requiring detailed records of final administration dates, dosages, and clearance timelines
- Individual variability factors such as metabolic considerations in research models can influence optimal washout duration
- Sequential research protocols demand careful planning to accommodate adequate washout periods between experimental phases
Understanding Retatrutide: Background and Mechanism
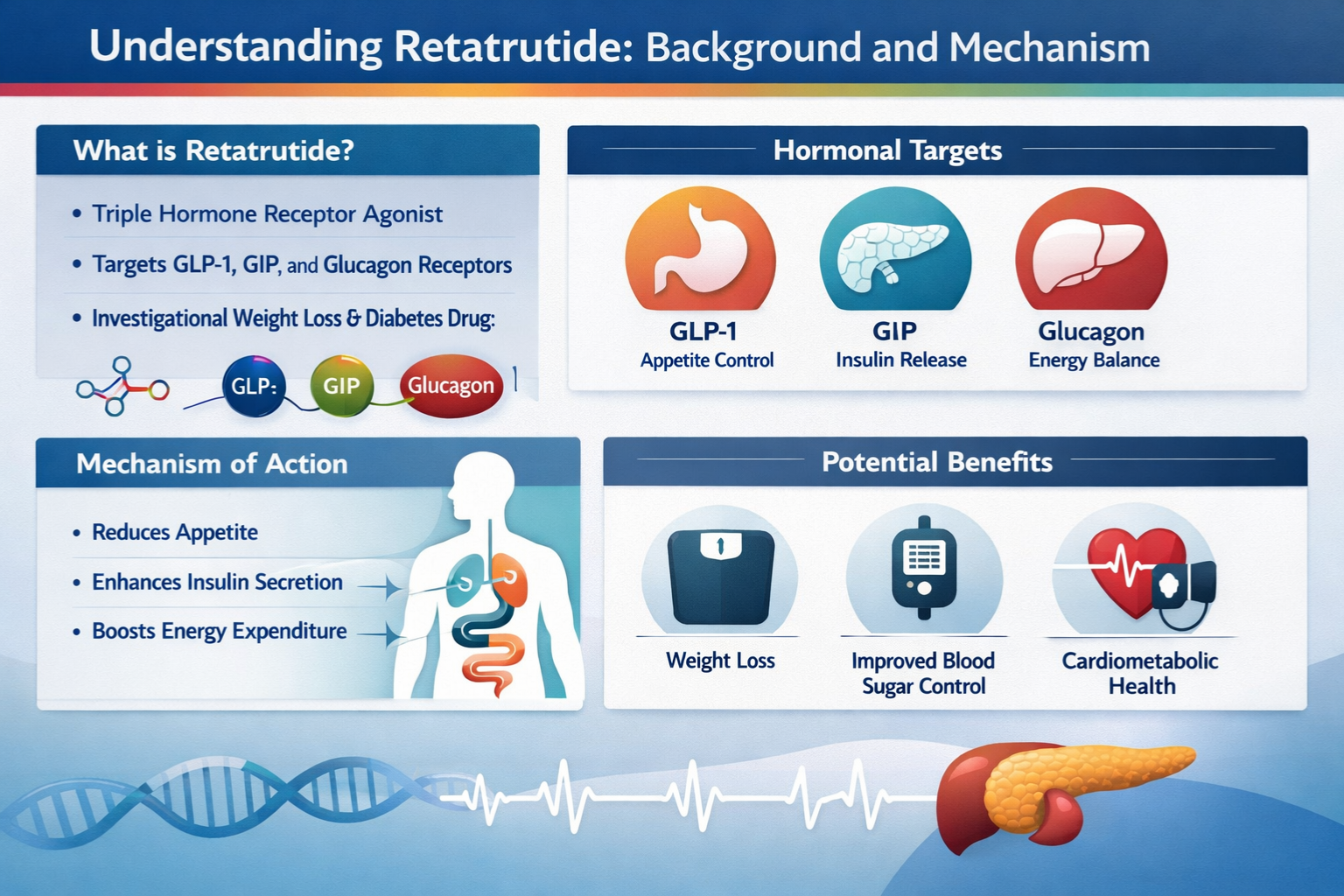
Retatrutide represents a significant advancement in peptide research, classified as a triple-agonist compound that simultaneously targets GIP (glucose-dependent insulinotropic polypeptide), GLP-1 (glucagon-like peptide-1), and glucagon receptors. This unique triple-mechanism distinguishes it from earlier dual-agonist compounds and has generated substantial interest within the research community.
For laboratories conducting investigations with research-grade peptides, understanding the fundamental properties of retatrutide is essential before establishing washout protocols. The compound’s molecular structure and receptor-binding characteristics create a pharmacological profile that differs markedly from single-target peptides.
Pharmacological Profile
The triple-agonist mechanism of retatrutide creates complex interactions within biological systems. Each receptor pathway contributes distinct effects:
- GIP receptor activation influences metabolic processes and cellular signaling
- GLP-1 receptor engagement affects multiple physiological pathways
- Glucagon receptor activity modulates energy regulation mechanisms
This multi-targeted approach results in a compound with extended biological activity and complex elimination kinetics—factors that directly impact washout period calculations.
Chemical Stability and Formulation
Research-grade retatrutide requires careful handling and storage to maintain compound integrity. The peptide’s stability profile influences both its biological half-life and the considerations for washout periods. Laboratories sourcing high-purity peptides must account for formulation factors when planning experimental timelines.
What Is a Washout Period and Why Does It Matter?
A washout period in research protocols refers to the designated timeframe during which a compound is allowed to clear from biological systems before initiating subsequent experimental phases. This interval serves multiple critical functions in maintaining research quality and validity.
Scientific Rationale for Washout Periods
The fundamental purpose of implementing a retatrutide washout period includes:
Elimination of Residual Effects 🧬
Complete clearance prevents interference with subsequent experimental protocols, ensuring that observed effects can be attributed to new interventions rather than lingering compound activity.
Baseline Re-establishment 📊
Adequate washout allows physiological parameters to return to baseline states, providing clean starting conditions for new research phases.
Prevention of Compound Interactions ⚗️
When transitioning between different peptide compounds or experimental conditions, proper washout prevents unexpected interactions that could confound results.
Data Integrity Protection 📋
Documented washout periods strengthen the validity of research findings and support reproducibility—cornerstones of quality scientific investigation.
Regulatory and Ethical Considerations
For research institutions and laboratories, implementing appropriate washout protocols demonstrates commitment to scientific rigor. Documentation of washout periods becomes part of the permanent research record, supporting:
- Protocol compliance and quality assurance
- Peer review processes and publication requirements
- Institutional oversight and research governance
- Transparent methodology for result interpretation
Retatrutide Washout Period: Recommended Duration and Guidelines
Establishing the appropriate retatrutide washout period requires careful consideration of multiple pharmacokinetic and experimental factors. Unlike shorter-acting peptides, retatrutide’s extended half-life necessitates longer clearance intervals.
Standard Washout Timeline
Based on current pharmacokinetic data and research protocols implemented across laboratory settings, the following guidelines represent evidence-based recommendations:
| Research Context | Minimum Washout | Recommended Washout | Extended Washout |
|---|---|---|---|
| Single-dose studies | 4-6 weeks | 6-8 weeks | 8-10 weeks |
| Repeated-dose protocols | 6-8 weeks | 8-10 weeks | 10-12 weeks |
| High-dose investigations | 8-10 weeks | 10-12 weeks | 12-16 weeks |
| Sequential compound studies | 8-12 weeks | 12-14 weeks | 14-16 weeks |
Calculating Washout Based on Half-Life
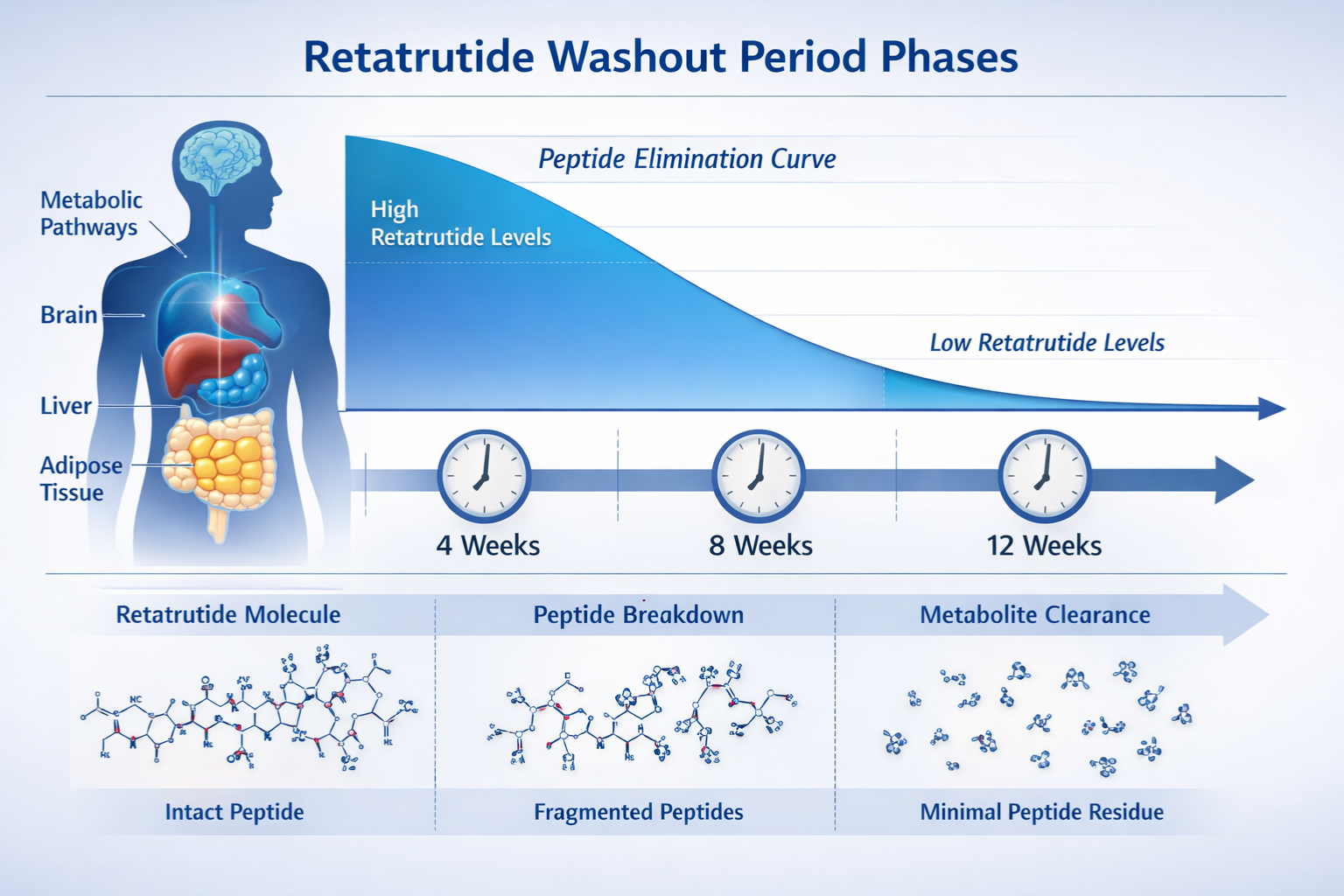
The pharmacokinetic principle underlying washout calculations centers on the compound’s elimination half-life. Retatrutide exhibits an approximate half-life of 6-7 days, significantly longer than many research peptides.
The Five Half-Life Rule ⏱️
A widely accepted principle in pharmacology suggests that approximately five half-lives are required to eliminate 97% of a compound from biological systems. For retatrutide:
- 1 half-life (6-7 days): ~50% elimination
- 2 half-lives (12-14 days): ~75% elimination
- 3 half-lives (18-21 days): ~87.5% elimination
- 4 half-lives (24-28 days): ~93.75% elimination
- 5 half-lives (30-35 days): ~97% elimination
This calculation suggests a minimum washout period of approximately 5-6 weeks for basic clearance. However, conservative research protocols typically extend this timeframe to account for:
- Individual variability in metabolic clearance
- Potential accumulation from repeated dosing
- Receptor occupancy duration beyond compound presence
- Downstream signaling effects that may persist
Dosage-Dependent Considerations
The retatrutide washout period should be adjusted based on the dosages used in research protocols:
Low-Dose Protocols (≤2mg)
Minimum 6-8 week washout typically sufficient for complete clearance and baseline restoration.
Moderate-Dose Protocols (2-8mg)
Recommended 8-10 week washout provides additional margin for complete elimination and physiological normalization.
High-Dose Protocols (>8mg)
Extended 10-12+ week washout accounts for potential accumulation effects and ensures complete clearance.
Research facilities working with compounds like Retatrutide 40mg should implement the longer washout intervals to ensure protocol integrity.
Factors Influencing Retatrutide Washout Duration
While standard guidelines provide essential frameworks, several variables can influence the optimal retatrutide washout period for specific research applications.
Metabolic Variability
Different research models exhibit varying metabolic rates that affect compound clearance:
- High metabolic activity may accelerate elimination, potentially reducing required washout duration
- Reduced metabolic function necessitates extended washout periods to ensure complete clearance
- Age-related factors in research models can significantly impact pharmacokinetic profiles
Researchers should consider baseline metabolic assessments when establishing washout timelines for their specific experimental conditions.
Frequency and Duration of Administration
The administration schedule significantly impacts washout requirements:
Single Administration
One-time dosing follows standard half-life calculations with minimal accumulation concerns.
Weekly Administration
Regular weekly dosing may create steady-state conditions where compound levels stabilize, requiring extended washout to account for accumulated exposure.
Multiple Daily Dosing
Frequent administration schedules (uncommon with retatrutide due to its long half-life) would necessitate the longest washout periods due to maximum accumulation potential.
Compound Purity and Formulation
The quality and purity of research-grade retatrutide can influence pharmacokinetic behavior. Laboratories sourcing from reputable suppliers offering high-purity research peptides benefit from consistent, predictable elimination profiles that support accurate washout planning.
Impurities or degradation products may exhibit different clearance rates, potentially extending the required washout period beyond standard calculations.
Sequential Research Protocol Design
When planning experiments that involve transitioning between different compounds, the retatrutide washout period must account for:
Cross-Reactivity Potential 🔬
If subsequent compounds target similar receptor pathways (such as GLP-1 agonists), extended washout ensures complete receptor availability and prevents competitive binding complications.
Additive or Synergistic Effects ⚡
Compounds with complementary mechanisms may produce unexpected interactions if insufficient washout occurs between protocols.
Biomarker Normalization 📈
Certain physiological markers affected by retatrutide may require additional time beyond compound clearance to return to baseline, necessitating extended washout periods.
Monitoring and Documenting the Washout Period
Proper documentation and monitoring of the retatrutide washout period represents essential laboratory practice for maintaining research integrity and supporting reproducible science.
Essential Documentation Elements
Comprehensive washout documentation should include:
✅ Final Administration Date and Time
Precise recording of when the last retatrutide dose was administered establishes the washout timeline starting point.
✅ Cumulative Dosage Information
Total exposure (dose × frequency × duration) provides context for washout duration decisions.
✅ Planned Washout Duration
Pre-specified washout period based on protocol requirements and pharmacokinetic considerations.
✅ Washout Completion Verification
Documented confirmation that the full washout period elapsed before initiating subsequent protocols.
✅ Baseline Parameter Assessment
Measurements confirming return to baseline conditions following washout completion.
Verification Methods
While direct measurement of retatrutide levels may not be feasible in all research settings, several approaches can verify adequate washout:
Physiological Parameter Monitoring 📊
Tracking relevant biomarkers and physiological measurements throughout the washout period can confirm return to baseline states.
Functional Assessments 🔍
Evaluating functional outcomes associated with retatrutide activity provides indirect evidence of compound clearance.
Standardized Observation Protocols
👁
️ Systematic observation and recording of relevant parameters throughout washout supports verification.
Record-Keeping Best Practices
Laboratory notebooks and electronic records should maintain clear, auditable documentation of washout periods. This documentation serves multiple purposes:
- Supporting internal quality assurance reviews
- Facilitating peer review and publication processes
- Enabling protocol replication by other researchers
- Demonstrating compliance with institutional guidelines
Comparing Retatrutide Washout to Other Research Peptides
Understanding how the retatrutide washout period compares to other commonly researched peptides provides valuable context for protocol planning.
Washout Comparison Table
| Peptide Compound | Approximate Half-Life | Typical Washout Period |
|---|---|---|
| Retatrutide | 6-7 days | 8-12 weeks |
| Semaglutide | 7 days | 8-10 weeks |
| Tirzepatide | 5 days | 6-8 weeks |
| BPC-157 | 4-6 hours | 1-2 weeks |
| TB-500 | 10 days | 6-8 weeks |
| CJC-1295 | 6-8 days | 6-8 weeks |
Why Retatrutide Requires Extended Washout
Several factors contribute to retatrutide’s requirement for relatively longer washout periods:
Triple-Receptor Mechanism
�
� The simultaneous engagement of three distinct receptor pathways creates more complex physiological effects that may persist beyond simple compound clearance.
Downstream Signaling Effects 🔄
Receptor activation initiates signaling cascades that can continue influencing cellular processes even after the compound itself has been eliminated.
Metabolic Adaptations ⚙️
The metabolic changes induced by retatrutide may require additional time to normalize fully, extending the functional washout period beyond pharmacokinetic predictions.
Research Safety Margins
🛡
️ Given retatrutide’s relatively recent emergence in research settings, conservative washout protocols provide additional safety margins until more extensive pharmacokinetic data becomes available.
Special Considerations for Sequential Research Protocols
Research programs involving multiple experimental phases or compound comparisons require particularly careful attention to retatrutide washout period planning.
Crossover Study Designs
Crossover research designs, where the same experimental subjects receive different treatments in sequence, demand rigorous washout protocols:
Adequate Separation ⏳
The washout period must be sufficient to prevent carryover effects that could confound results from subsequent treatment phases.
Order Effects 🔀
Even with adequate washout, researchers should consider counterbalancing treatment order to control for potential sequence effects.
Extended Monitoring 📅
Crossover designs may benefit from extended washout periods (12-16 weeks) to provide maximum confidence in treatment phase independence.
Transitioning Between Peptide Compounds
When research protocols involve transitioning from retatrutide to other peptides (or vice versa), additional considerations apply:
Mechanism Overlap Assessment 🔬
Evaluate the degree of mechanistic overlap between compounds. Transitioning from retatrutide to another GLP-1 agonist requires more conservative washout than switching to a mechanistically distinct compound.
Receptor Sensitivity Considerations 📡
Prolonged receptor activation by retatrutide might influence receptor sensitivity or expression, potentially affecting response to subsequent compounds even after complete retatrutide clearance.
Baseline Re-establishment Verification ✓
Before initiating the new compound, verify that relevant physiological parameters have returned to pre-retatrutide baseline levels.
Long-Term Research Program Planning
Multi-year research programs incorporating retatrutide should integrate washout periods into overall timeline planning:
- Annual protocol reviews should reassess washout adequacy based on accumulated data
- Resource allocation must account for non-active research periods during washout
- Publication timelines should incorporate washout durations to set realistic completion expectations
- Grant applications should include washout periods in proposed timelines and budgets
Common Questions About Retatrutide Washout Periods
Can the Washout Period Be Shortened?
While minimum washout guidelines exist, shortening the retatrutide washout period below recommended durations risks compromising research validity. Factors that might support shorter washout include:
- Single low-dose administration
- Extensive pharmacokinetic monitoring confirming complete elimination
- Research questions specifically examining shorter washout adequacy
- Metabolic assessments indicating accelerated clearance
However, most research protocols benefit from conservative washout approaches that prioritize data integrity over timeline compression.
What Happens If Washout Is Insufficient?
Inadequate washout periods can create several research complications:
Confounded Results ❌
Residual retatrutide effects may be misattributed to subsequent interventions, leading to incorrect conclusions.
Reduced Sensitivity 📉
Incomplete baseline restoration may diminish the ability to detect effects from new experimental conditions.
Interaction Effects ⚠️
Overlapping compound exposure may produce unexpected interactions that complicate interpretation.
Publication Challenges 📄
Insufficient washout documentation may raise concerns during peer review, potentially affecting publication prospects.
How Should Washout Be Documented?
Comprehensive documentation should include:
- Protocol specification of planned washout duration with scientific rationale
- Precise timestamps for final retatrutide administration
- Washout completion confirmation before initiating subsequent protocols
- Baseline assessments demonstrating physiological parameter normalization
- Any deviations from planned washout with justification
This documentation becomes part of the permanent research record and should be maintained according to institutional data retention policies.
Best Practices for Implementing Retatrutide Washout Protocols
Protocol Development Recommendations
When establishing retatrutide washout period protocols for research programs:
Consult Pharmacokinetic Literature 📚
Review available pharmacokinetic data for retatrutide, including half-life determinations, clearance rates, and elimination pathways.
Apply Conservative Margins
🛡
️ When uncertainty exists, implement longer washout periods. The cost of extended washout is typically minimal compared to the consequences of inadequate clearance.
Document Decision Rationale 📝
Record the scientific reasoning behind washout duration decisions, including calculations, literature citations, and safety considerations.
Establish Verification Procedures ✅
Define how washout completion will be verified, whether through biomarker assessment, functional testing, or timeline confirmation.
Plan for Contingencies 🔄
Develop protocols for addressing situations where baseline parameters don’t normalize within expected washout timeframes.
Quality Assurance Integration
Washout period compliance should be integrated into broader laboratory quality management:
- Standard Operating Procedures (SOPs) should specify washout requirements for retatrutide protocols
- Protocol review processes should verify adequate washout planning before research initiation
- Data management systems should flag attempts to initiate new protocols before washout completion
- Training programs should educate research staff on washout importance and documentation requirements
Collaboration with Peptide Suppliers
Establishing relationships with reputable peptide suppliers supports washout protocol implementation. Suppliers like PEPTIDE PRO can provide:
- Certificate of Analysis (COA) documentation supporting purity verification
- Storage and handling guidance that influences compound stability and pharmacokinetics
- Technical consultation regarding compound properties relevant to washout planning
- Consistent product quality enabling predictable pharmacokinetic behavior across research batches
Future Directions and Emerging Research
As research with retatrutide expands throughout 2026 and beyond, our understanding of optimal retatrutide washout period protocols continues to evolve.
Ongoing Pharmacokinetic Studies
Current research efforts are working to refine washout recommendations through:
Population Pharmacokinetic Modeling 📊
Advanced modeling approaches that account for variability across different research models and conditions.
Biomarker Development 🔬
Identification of specific biomarkers that can directly or indirectly indicate retatrutide clearance status.
Long-Term Effect Studies ⏱️
Investigation of whether retatrutide produces persistent effects that extend beyond compound elimination.
Personalized Washout Approaches
Future protocols may incorporate more individualized washout determinations based on:
- Metabolic profiling of specific research models
- Real-time monitoring of relevant physiological parameters
- Computational modeling predicting clearance based on dosing history
- Genetic or phenotypic factors influencing pharmacokinetics
Standardization Efforts
The research community continues working toward standardized washout protocols that balance scientific rigor with practical feasibility. Professional organizations and research consortia are developing consensus guidelines that will inform best practices across institutions.
Conclusion: Ensuring Research Integrity Through Proper Washout Protocols
The retatrutide washout period represents far more than a procedural checkbox in research protocols—it serves as a fundamental safeguard for scientific validity and data integrity. As laboratories across the UK and internationally expand their investigations into this promising triple-agonist peptide, implementing appropriate washout protocols becomes increasingly critical.
Key Implementation Points
✅ Standard washout duration: 8-12 weeks for most research applications
✅ Conservative approach: Extend washout periods when uncertainty exists
✅ Comprehensive documentation: Maintain detailed records supporting protocol compliance
✅ Quality assurance integration: Incorporate washout verification into laboratory quality systems
✅ Ongoing assessment: Review and update protocols as new pharmacokinetic data emerges
Taking Action
For research teams planning retatrutide investigations:
- Review your current protocols to ensure adequate washout periods are specified
- Establish documentation systems that capture essential washout information
- Source high-quality compounds from reputable suppliers committed to research-grade purity
- Integrate washout planning into overall research timelines and resource allocation
- Stay informed about emerging pharmacokinetic data that may refine washout recommendations
Supporting Your Research Success
Quality research begins with quality compounds and rigorous protocols. PEPTIDE PRO provides the research-grade peptides, professional service, and technical support that laboratories depend on for conducting cutting-edge investigations. With same-day dispatch for orders placed before 1pm Monday-Friday, comprehensive product documentation, and a commitment to exceptional purity, PEPTIDE PRO supports researchers in maintaining the highest standards of scientific excellence.
Whether you’re establishing new retatrutide protocols or refining existing research programs, proper attention to washout periods protects your investment in time, resources, and scientific credibility. The additional weeks allocated to adequate washout represent a small fraction of total research timelines but provide invaluable assurance that your findings reflect true experimental effects rather than confounding carryover.
For questions about retatrutide washout protocols, compound selection, or research planning, the team at PEPTIDE PRO stands ready to provide professional guidance supporting your research objectives. Contact us to discuss your specific research requirements and discover how our commitment to quality, consistency, and service can support your scientific endeavors.
Disclaimer: All peptides supplied by PEPTIDE PRO are strictly for research use only. Not for human or animal consumption. Researchers should follow all applicable institutional guidelines, regulatory requirements, and ethical standards when conducting peptide research.
