The landscape of metabolic research continues to evolve rapidly, and among the most promising compounds under investigation in 2026 is LY3437943 retatrutide—a novel triple receptor agonist that has captured significant attention within the scientific community. This sophisticated research peptide represents a groundbreaking approach to understanding metabolic regulation through its unique mechanism targeting three distinct pathways simultaneously. For researchers and laboratories seeking to explore cutting-edge metabolic compounds, understanding the properties, mechanisms, and research applications of LY3437943 retatrutide has become increasingly essential.
Key Takeaways
- LY3437943 retatrutide is a triple receptor agonist targeting GIP, GLP-1, and glucagon receptors simultaneously for comprehensive metabolic research
- This research peptide demonstrates unique pharmacological properties distinct from dual agonists like tirzepatide and semaglutide
- Proper handling, reconstitution, and storage protocols are critical for maintaining peptide integrity in laboratory settings
- High-purity research-grade retatrutide is available through specialized suppliers like PEPTIDE PRO for qualified research applications
- Understanding molecular structure and receptor interactions is fundamental to designing effective research protocols with this compound
🔬 What is LY3437943 Retatrutide?
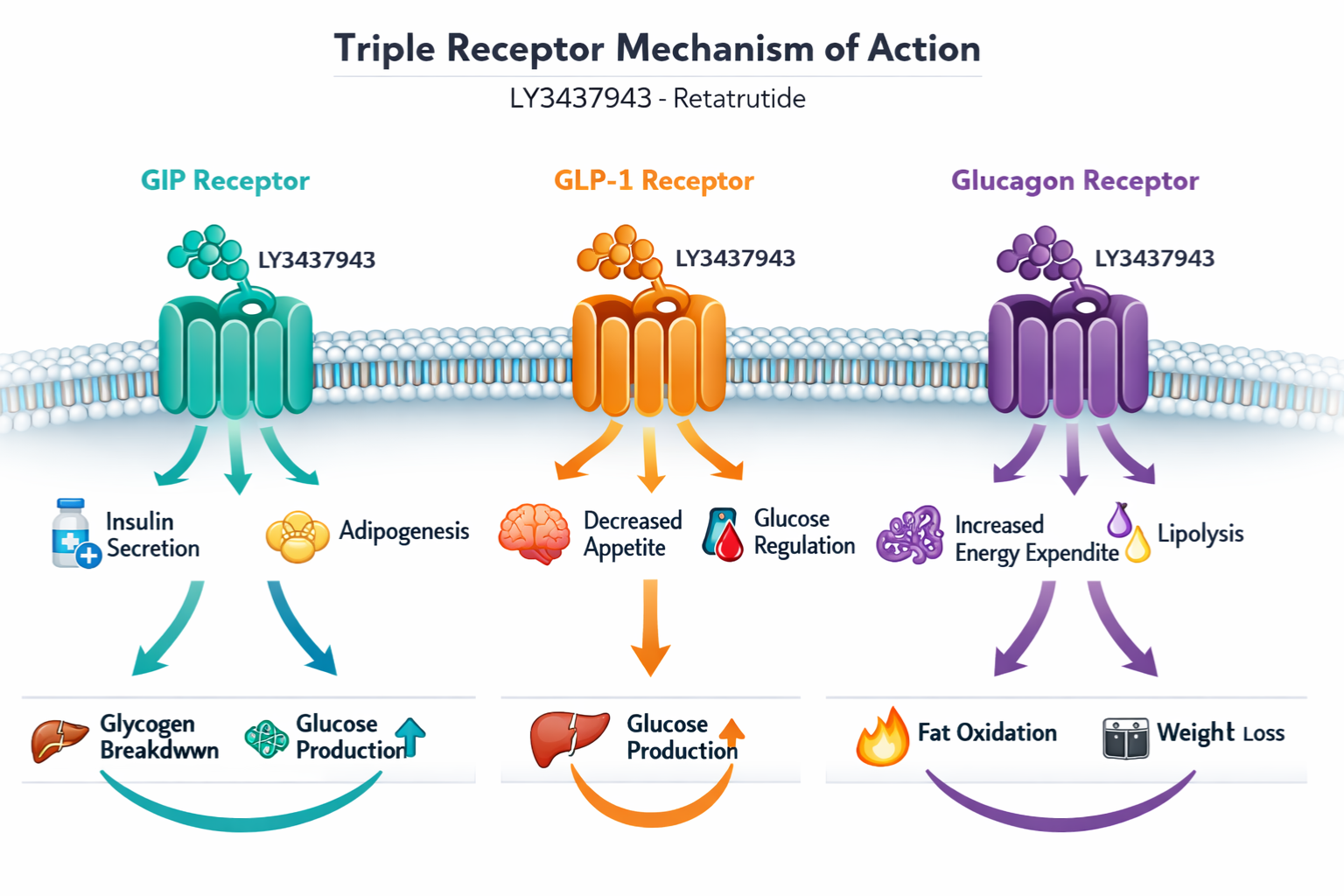
LY3437943 retatrutide is a synthetic peptide compound developed as a triple agonist that simultaneously activates three metabolic hormone receptors: the glucose-dependent insulinotropic polypeptide (GIP) receptor, the glucagon-like peptide-1 (GLP-1) receptor, and the glucagon receptor. This triple mechanism of action distinguishes retatrutide from earlier dual-agonist compounds and represents a significant advancement in metabolic research peptide development.
Molecular Characteristics and Structure
The molecular structure of LY3437943 retatrutide has been carefully engineered to achieve balanced activation across all three target receptors. This research compound features:
- Molecular Formula: Precisely designed peptide sequence optimized for receptor binding
- Molecular Weight: Approximately 4,800-5,200 Da (depending on formulation)
- Physical Form: Typically supplied as lyophilized white to off-white powder
- Solubility: Soluble in sterile water and appropriate reconstitution solutions
- Stability: Requires controlled temperature storage to maintain structural integrity
The peptide’s unique structure allows it to engage with three distinct receptor families, creating a comprehensive metabolic signaling profile that has made it a valuable tool for researchers investigating complex metabolic pathways.
Development Background and Research Context
Originally designated as LY3437943 during early development phases, retatrutide emerged from systematic research efforts to create more comprehensive metabolic modulators. The compound builds upon knowledge gained from earlier incretin-based research peptides while introducing the novel glucagon receptor agonism component.
Research institutions worldwide have incorporated LY3437943 retatrutide into their investigational protocols to better understand:
- Multi-receptor signaling dynamics
- Metabolic pathway interactions
- Energy homeostasis mechanisms
- Glucose and lipid metabolism coordination
- Receptor cross-talk phenomena
For laboratories seeking to acquire research-grade retatrutide, PEPTIDE PRO offers high-purity formulations with comprehensive quality documentation.
⚙️ Mechanism of Action: Triple Receptor Agonism
The defining characteristic of LY3437943 retatrutide is its unprecedented triple agonist mechanism. Understanding how this compound interacts with three distinct receptor systems is fundamental to designing appropriate research protocols and interpreting experimental outcomes.
GIP Receptor Activation
The glucose-dependent insulinotropic polypeptide (GIP) receptor represents the first component of retatrutide’s triple action. GIP receptor activation in research models has been associated with:
- Enhanced glucose-dependent insulin secretion
- Potential effects on lipid metabolism
- Influences on adipose tissue function
- Modulation of energy storage pathways
Retatrutide’s GIP receptor agonism provides researchers with a tool to investigate how this often-overlooked incretin pathway contributes to overall metabolic regulation.
GLP-1 Receptor Activation
The glucagon-like peptide-1 (GLP-1) receptor pathway is well-established in metabolic research. LY3437943 retatrutide’s GLP-1 component enables investigation of:
- Glucose-dependent insulin release mechanisms
- Gastric emptying modulation
- Central nervous system satiety signaling
- Pancreatic beta-cell function
- Glucagon secretion suppression
Researchers familiar with semaglutide or other GLP-1 agonists will recognize these pathways, though retatrutide’s multi-receptor approach creates distinct pharmacological profiles.
Glucagon Receptor Activation
The third and most distinctive element of retatrutide’s mechanism is glucagon receptor agonism. This component differentiates it from dual agonists like tirzepatide and enables research into:
- Energy expenditure enhancement
- Hepatic glucose output regulation
- Lipid oxidation pathways
- Thermogenic responses
- Metabolic rate modulation
The balanced integration of glucagon agonism with GIP and GLP-1 activation creates a unique pharmacological profile that researchers can leverage to investigate complex metabolic interactions.
Synergistic Effects and Research Implications
The simultaneous activation of all three receptors creates potential synergistic effects that cannot be replicated by single-agonist compounds or simple combinations. Research applications include:
| Receptor System | Primary Research Areas | Potential Synergies |
|---|---|---|
| GIP | Insulin secretion, lipid metabolism | Enhanced glucose disposal when combined with GLP-1 |
| GLP-1 | Satiety, insulin release, gastric emptying | Complementary effects with GIP on beta-cell function |
| Glucagon | Energy expenditure, hepatic metabolism | Counterbalances anabolic effects, increases metabolic rate |
This multi-pathway approach makes LY3437943 retatrutide particularly valuable for researchers investigating:
- ✅ Comprehensive metabolic regulation
- ✅ Energy balance mechanisms
- ✅ Multi-system metabolic disorders
- ✅ Receptor interaction dynamics
- ✅ Novel therapeutic target identification
📊 Research Applications and Laboratory Uses
LY3437943 retatrutide has found application across numerous research domains in 2026, with laboratories worldwide incorporating this triple agonist into diverse investigational protocols. Understanding the breadth of potential research applications helps scientists determine whether this compound aligns with their specific research objectives.
Metabolic Research Studies
The primary application domain for LY3437943 retatrutide remains metabolic research, where its triple mechanism provides unique investigational opportunities:
Glucose Homeostasis Research
- Investigation of multi-pathway glucose regulation
- Studies on insulin secretion dynamics
- Examination of hepatic glucose production
- Analysis of peripheral glucose uptake mechanisms
- Research into glucose-dependent hormonal responses
Lipid Metabolism Studies
- Exploration of fatty acid oxidation pathways
- Investigation of adipose tissue metabolism
- Studies on hepatic lipid handling
- Research into lipid storage and mobilization
- Analysis of lipid-glucose interaction dynamics
Energy Balance Research
- Investigation of energy expenditure mechanisms
- Studies on thermogenic pathway activation
- Research into metabolic rate modulation
- Examination of energy intake regulation
- Analysis of energy partitioning between tissues
Receptor Pharmacology Investigations
Beyond metabolic applications, LY3437943 retatrutide serves as a valuable tool for fundamental receptor pharmacology research:
- Receptor Binding Studies: Investigating binding affinities, kinetics, and selectivity across GIP, GLP-1, and glucagon receptors
- Signal Transduction Research: Examining downstream signaling cascades activated by multi-receptor engagement
- Receptor Desensitization: Studying long-term receptor responses to sustained agonist exposure
- Cross-Talk Mechanisms: Investigating how simultaneous activation of multiple pathways influences cellular responses
Comparative Pharmacology Studies
Researchers frequently use retatrutide in comparative studies alongside related compounds:
- Comparison with dual agonists like tirzepatide
- Evaluation against single agonists such as semaglutide
- Assessment relative to other metabolic research peptides
- Structure-activity relationship investigations
- Dose-response comparative analyses
Experimental Protocol Considerations
When designing research protocols incorporating LY3437943 retatrutide, investigators should consider:
Dosing Parameters
- Concentration ranges appropriate for in vitro vs. in vivo models
- Frequency of administration in longitudinal studies
- Dose-response relationship characterization
- Comparative dosing relative to other agonists
Timing Considerations
- Onset of receptor activation
- Duration of biological effects
- Optimal sampling timepoints for endpoint measurements
- Washout periods for sequential experiments
Control Groups
- Vehicle-only controls
- Single-agonist comparators
- Dual-agonist comparators
- Receptor-specific antagonist co-treatment groups
For laboratories requiring additional metabolic research compounds for comparative studies, PEPTIDE PRO’s research peptide catalogue offers an extensive selection of high-purity options.
🧪 Reconstitution, Handling, and Storage Protocols
Proper handling of LY3437943 retatrutide is essential for maintaining peptide integrity and ensuring reliable, reproducible research outcomes. Research-grade peptides require careful attention to reconstitution procedures, storage conditions, and handling practices.
Pre-Reconstitution Storage
Lyophilized LY3437943 retatrutide should be stored under specific conditions to preserve stability:
- Temperature: Store at -20°C to -80°C for long-term storage
- Protection: Keep protected from light exposure
- Humidity: Store in low-humidity environment
- Duration: Lyophilized peptide typically stable for 12-24 months when properly stored
- Container: Keep in original sealed container until ready for use
Professional Tip: Always allow vials to reach room temperature before opening to prevent condensation formation, which can degrade the peptide.
Reconstitution Procedures
Proper reconstitution is critical for achieving accurate concentrations and maintaining peptide stability:
Recommended Reconstitution Solutions
- Sterile water (most common)
- Bacteriostatic water (for multi-dose applications)
- Sterile saline (0.9% NaCl)
- Buffered solutions (pH 7.0-7.4) for specific applications
Reconstitution Steps
- Prepare workspace: Ensure clean, sterile working environment
- Calculate volume: Determine appropriate volume for desired concentration
- Add solvent slowly: Direct solvent against vial wall, not directly onto peptide
- Gentle mixing: Swirl gently; avoid vigorous shaking or vortexing
- Complete dissolution: Allow adequate time for complete dissolution (typically 5-10 minutes)
- Visual inspection: Verify solution is clear without visible particles
- Label clearly: Mark vial with compound name, concentration, date, and researcher initials
Example Concentration Calculation
For a 10mg vial of retatrutide:
- To achieve 1mg/mL concentration: Add 10mL reconstitution solution
- To achieve 2mg/mL concentration: Add 5mL reconstitution solution
- To achieve 5mg/mL concentration: Add 2mL reconstitution solution
Post-Reconstitution Storage
Once reconstituted, LY3437943 retatrutide requires different storage conditions:
| Storage Condition | Temperature | Typical Stability | Best Use Case |
|---|---|---|---|
| Short-term | 2-8°C (refrigerated) | 7-14 days | Ongoing experiments |
| Medium-term | -20°C (frozen) | 30-60 days | Intermittent use |
| Long-term | -80°C (deep frozen) | 3-6 months | Extended studies |
Important Considerations
- ⚠️ Avoid repeated freeze-thaw cycles, which can degrade peptide structure
- ⚠️ Aliquot into single-use portions when possible
- ⚠️ Protect from light exposure during storage
- ⚠️ Monitor for any precipitation or cloudiness before use
Handling Best Practices
To maintain research integrity and peptide quality:
Laboratory Safety
- Wear appropriate personal protective equipment (gloves, lab coat, safety glasses)
- Work in appropriate containment (biosafety cabinet when indicated)
- Follow institutional safety protocols
- Dispose of materials according to laboratory waste procedures
Quality Control
- Document all reconstitution procedures
- Record storage conditions and dates
- Monitor appearance before each use
- Maintain chain of custody documentation
- Retain Certificates of Analysis from supplier
Contamination Prevention
- Use sterile technique throughout
- Employ single-use sterile syringes and needles
- Avoid touching vial openings or solution surfaces
- Work in clean, organized laboratory space
- Minimize exposure time to ambient conditions
Research institutions can obtain detailed handling protocols and Certificates of Analysis when purchasing from reputable suppliers like PEPTIDE PRO.
📈 Comparison with Related Research Peptides
Understanding how LY3437943 retatrutide compares to related metabolic research peptides helps researchers select the most appropriate compound for their specific investigational objectives. The metabolic peptide landscape in 2026 includes several important compounds, each with distinct characteristics.
Retatrutide vs. Tirzepatide
Tirzepatide represents the most closely related compound to retatrutide, functioning as a dual GIP/GLP-1 receptor agonist:
Key Differences
| Characteristic | LY3437943 Retatrutide | Tirzepatide |
|---|---|---|
| Receptor Targets | GIP + GLP-1 + Glucagon | GIP + GLP-1 |
| Mechanism | Triple agonist | Dual agonist |
| Glucagon Activity |
✅ Yes |
❌ No | | Energy Expenditure | Enhanced via glucagon | Limited | | Research Applications | Broader metabolic scope | Focused on incretin pathways |
Research Implications
- Retatrutide offers additional glucagon-mediated effects not present in tirzepatide
- Tirzepatide provides cleaner dual-pathway investigation without glucagon confounds
- Both compounds share GIP/GLP-1 mechanisms, enabling direct comparison studies
- Retatrutide may demonstrate enhanced metabolic rate effects due to glucagon component
Retatrutide vs. Semaglutide
Semaglutide functions as a selective GLP-1 receptor agonist, representing a single-pathway approach:
Comparative Profile
Semaglutide Characteristics
- Highly selective GLP-1 receptor agonism
- Well-characterized pharmacology
- Extensive research literature available
- Longer half-life due to albumin binding
- Established dosing protocols
Retatrutide Advantages for Research
- Multi-pathway investigation capability
- Broader metabolic effects profile
- Novel mechanism for exploratory studies
- Potential synergistic receptor interactions
- Glucagon and GIP pathway access
When to Choose Each Compound
- Choose Semaglutide: For isolated GLP-1 pathway research, when extensive literature comparison is needed, or for well-established protocols
- Choose Retatrutide: For comprehensive metabolic studies, multi-receptor investigations, or novel exploratory research
Retatrutide vs. Other Triple/Multi-Agonists
The multi-agonist research peptide field includes several emerging compounds:
Survodutide
- Dual GLP-1/glucagon agonist
- Lacks GIP component present in retatrutide
- Different balance of receptor activation
- Available through specialized suppliers like PEPTIDE PRO
Mazdutide
- Another dual GLP-1/glucagon agonist
- Alternative to survodutide with distinct pharmacology
- Useful for comparative studies with retatrutide
- Research-grade formulations available
Cagrisema (Cagrilintide + Semaglutide)
- Combination of amylin analog and GLP-1 agonist
- Different mechanism from retatrutide’s triple agonism
- Provides alternative multi-pathway approach
- Available for comparative research
Selecting the Appropriate Research Compound
Researchers should consider several factors when choosing between LY3437943 retatrutide and related peptides:
Research Objectives
�
� Single-pathway investigation → Semaglutide or other selective agonists
�
� Dual-pathway studies → Tirzepatide or dual agonists
�
� Comprehensive metabolic research → Retatrutide
�
� Glucagon-specific effects → Retatrutide or glucagon-containing dual agonists
Experimental Design
- Comparative studies benefit from having multiple related compounds
- Mechanism-of-action research requires compounds with different receptor profiles
- Dose-response studies should consider potency differences
- Temporal studies must account for pharmacokinetic variations
Resource Considerations
- Literature availability for each compound
- Cost and availability through research suppliers
- Established protocols vs. novel method development
- Analytical capabilities for measuring endpoints
🔍 Quality Considerations and Sourcing Research-Grade Retatrutide
The quality of LY3437943 retatrutide used in research directly impacts experimental validity, reproducibility, and scientific conclusions. Understanding quality parameters and sourcing considerations is essential for maintaining research integrity.
Purity Standards and Specifications
Research-grade peptides should meet stringent purity criteria:
Purity Levels
- ≥95% purity: Minimum standard for basic research applications
- ≥98% purity: Recommended for most research protocols
- ≥99% purity: Required for high-precision studies and pharmacokinetic research
Purity Assessment Methods
- High-Performance Liquid Chromatography (HPLC)
- Mass Spectrometry (MS)
- Amino Acid Analysis (AAA)
- Nuclear Magnetic Resonance (NMR) for structural verification
Certificates of Analysis (COA)
Reputable suppliers provide comprehensive Certificates of Analysis documenting:
Essential COA Components
- ✅ Batch/lot number
- ✅ Purity percentage and analysis method
- ✅ Molecular weight confirmation
- ✅ Peptide content (mg per vial)
- ✅ Storage recommendations
- ✅ Expiration or retest date
- ✅ Manufacturing date
- ✅ Quality control test results
Research Best Practice: Always request and retain COAs for all peptide batches used in studies. This documentation is essential for publication, regulatory compliance, and troubleshooting experimental issues.
Supplier Selection Criteria
When sourcing LY3437943 retatrutide for research applications, evaluate suppliers based on:
Quality Assurance
- Documented quality control procedures
- Third-party testing verification
- Consistent batch-to-batch quality
- Comprehensive product documentation
- Transparent manufacturing information
Service and Support
- Technical support availability
- Prompt response to inquiries
- Knowledgeable staff familiar with research applications
- Custom formulation capabilities when needed
- Reliable customer service
Logistics and Delivery
- Appropriate temperature-controlled shipping
- Fast delivery options for time-sensitive research
- International shipping capabilities
- Order tracking systems
- Reliable inventory availability
Regulatory Compliance
- Clear “For Research Use Only” labeling
- Compliance with relevant regulations
- Proper documentation for institutional requirements
- Ethical sourcing and handling practices
PEPTIDE PRO: Trusted Source for Research-Grade Retatrutide
PEPTIDE PRO has established itself as a reliable supplier of high-purity research peptides, including LY3437943 retatrutide, offering several advantages for research institutions:
Quality Commitment
- Research-grade peptides with documented purity ≥98%
- Comprehensive Certificates of Analysis for every batch
- Strict quality control under controlled conditions
- Consistent, reliable product specifications
Researcher-Focused Service
- Fast UK delivery with same-day dispatch for orders before 1pm (Monday-Friday)
- International shipping options for global research community
- Professional customer support with technical knowledge
- Transparent product information and storage guidance
Extensive Catalogue
- Broad selection of metabolic research peptides
- Related compounds for comparative studies
- Regular addition of new research compounds
- Clearly labeled “For Research Use Only” products
Professional Standards
- Secure checkout supporting multiple currencies (GBP, EUR, USD)
- Temperature-appropriate packaging for peptide stability
- Full tracking options for all orders
- Established reputation within UK and international research community
Researchers can explore the complete range of available compounds through the PEPTIDE PRO research peptide catalogue.
Storage and Handling Upon Receipt
When receiving LY3437943 retatrutide from suppliers:
Immediate Actions
- Verify package condition and temperature indicators
- Confirm product identity and batch number
- Check COA against product received
- Transfer to appropriate storage immediately
- Document receipt date and storage location
- Inspect for any visible damage or contamination
- Record in laboratory inventory system
Quality Verification
- Visual inspection of lyophilized peptide (should be white to off-white powder)
- Verify seal integrity on vial
- Check labeling accuracy and completeness
- Confirm expiration or retest date
- Review COA for any quality concerns
📚 Research Protocol Development with LY3437943 Retatrutide
Developing robust research protocols incorporating LY3437943 retatrutide requires careful consideration of experimental design, controls, endpoints, and analytical methods. This section provides guidance for researchers planning studies with this triple agonist peptide.
Experimental Design Considerations
Study Objectives Definition Before initiating research with retatrutide, clearly define:
- Primary research questions
- Specific hypotheses to test
- Expected outcomes and endpoints
- Mechanistic questions to address
- Comparative elements (if any)
Model System Selection
Researchers must choose appropriate experimental models:
In Vitro Systems
- Cell culture models expressing target receptors
- Isolated tissue preparations
- Receptor binding assays
- Signal transduction studies
- High-throughput screening applications
Ex Vivo Systems
- Isolated organ preparations
- Tissue slice cultures
- Primary cell isolations
- Organoid systems
In Vivo Models
- Appropriate species selection based on receptor homology
- Consideration of pharmacokinetic differences
- Ethical approval and institutional compliance
- Proper animal care and monitoring protocols
Dosing Strategy Development
Establishing appropriate dosing for LY3437943 retatrutide research requires:
Dose-Response Characterization
- Initial dose-finding studies across wide concentration range
- Identification of EC50 values for each receptor pathway
- Determination of maximum effective concentrations
- Assessment of dose-dependent effects
- Comparison with reference compounds
Concentration Calculations
For in vitro studies:
- Consider receptor expression levels in chosen cell system
- Account for media volume and binding kinetics
- Plan for concentration range spanning 3-4 log units
- Include sub-threshold and supra-maximal concentrations
For in vivo studies:
- Consider species-specific pharmacokinetics
- Account for bioavailability and distribution
- Plan for dose escalation studies
- Monitor for dose-limiting factors
Timing Considerations
- Acute vs. chronic exposure protocols
- Frequency of administration
- Washout periods between doses
- Optimal timepoints for endpoint measurements
- Consideration of receptor desensitization
Control Groups and Comparators
Rigorous research with LY3437943 retatrutide requires appropriate controls:
Essential Control Groups
- Vehicle control: Same volume/composition without peptide
- Baseline measurements: Pre-treatment values
- Negative controls: Inactive analogs or scrambled sequences
- Positive controls: Known effective compounds
Comparative Compounds
- Single agonists (semaglutide for GLP-1 comparison)
- Dual agonists (tirzepatide for GIP/GLP-1 comparison)
- Alternative triple agonists (when available)
- Receptor-specific antagonists for mechanism validation
Endpoint Selection and Measurement
Primary Endpoints
Choose endpoints that directly address research objectives:
Receptor Activation Endpoints
- cAMP accumulation assays
- Calcium mobilization measurements
- Receptor internalization studies
- Downstream signaling pathway activation
- Gene expression changes
Metabolic Endpoints
- Glucose uptake measurements
- Insulin secretion assays
- Lipid metabolism markers
- Energy expenditure assessments
- Substrate oxidation rates
Physiological Endpoints
- Body composition changes
- Food intake measurements
- Metabolic rate determinations
- Tissue-specific effects
- Systemic metabolic parameters
Secondary and Exploratory Endpoints
- Mechanistic biomarkers
- Safety/tolerability parameters
- Pharmacokinetic measurements
- Tissue distribution studies
- Long-term adaptation responses
Data Collection and Analysis
Experimental Rigor
- Adequate sample sizes based on power calculations
- Randomization of treatment assignments
- Blinding of investigators when feasible
- Standardized measurement procedures
- Quality control throughout data collection
Statistical Considerations
- Pre-specified statistical analysis plan
- Appropriate statistical tests for data type
- Correction for multiple comparisons
- Handling of missing data
- Sensitivity analyses
Documentation Requirements
- Detailed protocol documentation
- Standard operating procedures (SOPs)
- Raw data retention
- Analysis code/methods preservation
- Comprehensive laboratory notebooks
Troubleshooting Common Issues
Variable Results
- Verify peptide quality and storage conditions
- Confirm receptor expression in model system
- Check for batch-to-batch variability
- Assess experimental technique consistency
- Review timing of measurements
Unexpected Outcomes
- Consider off-target effects
- Evaluate dose appropriateness
- Assess model system suitability
- Review literature for similar findings
- Consult with colleagues or technical support
Technical Challenges
- Peptide solubility issues → Adjust reconstitution solution
- Stability concerns → Optimize storage and handling
- Detection sensitivity → Enhance analytical methods
- Reproducibility problems → Standardize protocols rigorously
🌐 Current Research Landscape and Future Directions (2026)
The research landscape surrounding LY3437943 retatrutide continues to expand in 2026, with growing scientific interest in triple agonist mechanisms and their potential applications across diverse research domains.
Emerging Research Themes
Multi-Receptor Pharmacology
The unique triple agonist profile of retatrutide has catalyzed broader interest in:
- Polypharmacology approaches to complex diseases
- Synergistic receptor interactions
- Balanced multi-pathway modulation
- Systems biology approaches to drug discovery
- Network pharmacology principles
Metabolic Systems Research
Researchers are leveraging retatrutide to investigate:
- Integrated metabolic regulation across tissues
- Organ-organ communication in metabolism
- Circadian regulation of metabolic pathways
- Metabolic flexibility and adaptation
- Precision nutrition and metabolism
Translational Research Applications
The research community is exploring:
- Biomarker identification for metabolic interventions
- Predictive models for treatment response
- Personalized medicine approaches
- Combination strategies with other interventions
- Long-term metabolic health optimization
Comparative Effectiveness Research
As the field matures, comparative studies are becoming increasingly sophisticated:
Head-to-Head Comparisons
- Retatrutide vs. dual agonists in matched protocols
- Triple agonist vs. combination therapy approaches
- Dose-response comparisons across compound classes
- Temporal effect profiles of different agonist types
Mechanism-Based Comparisons
- Receptor-specific contribution studies using selective antagonists
- Pathway analysis comparing single, dual, and triple agonists
- Tissue-specific effects across different agonist profiles
- Metabolic phenotyping with different receptor combinations
Technological Advances Enabling Retatrutide Research
Analytical Technologies
- Advanced mass spectrometry for peptide characterization
- High-resolution imaging for tissue distribution studies
- Multi-omics approaches (genomics, proteomics, metabolomics)
- Real-time biosensors for metabolic monitoring
- Artificial intelligence for data analysis and pattern recognition
Model Systems
- Human-relevant cell culture models (iPSC-derived cells, organoids)
- Humanized animal models with human receptor expression
- Microfluidic organ-on-chip systems
- 3D tissue culture platforms
- CRISPR-based receptor modification systems
Research Challenges and Opportunities
Current Challenges
Researchers working with LY3437943 retatrutide face several ongoing challenges:
Complexity of Multi-Receptor Systems
- Difficult to isolate individual receptor contributions
- Complex pharmacokinetic/pharmacodynamic relationships
- Tissue-specific receptor expression variations
- Temporal dynamics of multi-pathway activation
Translational Gaps
- Species differences in receptor pharmacology
- Model system limitations
- Long-term effects assessment
- Individual variability in responses
Technical Limitations
- Analytical method sensitivity for some endpoints
- Real-time monitoring of multiple pathways simultaneously
- Standardization across laboratories
- Cost and availability of advanced technologies
Future Opportunities
The research community has identified numerous opportunities for advancing retatrutide research:
Mechanistic Understanding
- 🔬 Detailed receptor structure-function studies
- 🔬 Allosteric modulation and receptor cross-talk
- 🔬 Tissue-specific signaling profiles
- 🔬 Temporal dynamics of receptor activation
- 🔬 Integration with other metabolic pathways
Novel Applications
- 🔬 Beyond traditional metabolic research
- 🔬 Neurometabolic investigations
- 🔬 Aging and longevity research
- 🔬 Metabolic aspects of other disease processes
- 🔬 Preventive medicine approaches
Methodological Innovations
- 🔬 Development of retatrutide-specific assays
- 🔬 Biomarker discovery and validation
- 🔬 Computational modeling of multi-receptor systems
- 🔬 High-throughput screening adaptations
- 🔬 Advanced imaging techniques
Collaborative Research Networks
The complexity of triple agonist research has fostered collaborative approaches:
Multi-Institutional Studies
- Coordinated research protocols across multiple sites
- Shared resources and expertise
- Larger sample sizes and statistical power
- Diverse model systems and approaches
- Accelerated knowledge generation
Interdisciplinary Teams
- Integration of pharmacologists, physiologists, biochemists
- Computational scientists and data analysts
- Clinicians providing translational perspective
- Industry partnerships for compound development
- Technology specialists for advanced methods
Regulatory and Ethical Considerations
Research with LY3437943 retatrutide must adhere to:
Research Use Only Designation
- Clearly labeled “For Research Use Only”
- Not approved for human consumption
- Appropriate institutional oversight
- Ethical research conduct principles
- Proper documentation and reporting
Laboratory Safety
- Appropriate handling procedures
- Personal protective equipment use
- Waste disposal protocols
- Emergency response procedures
- Training and competency requirements
Data Integrity
- Rigorous documentation practices
- Transparent reporting of methods and results
- Reproducibility standards
- Data sharing when appropriate
- Conflict of interest disclosure
�
� Practical Recommendations for Researchers
For scientists planning to incorporate LY3437943 retatrutide into their research programs, these practical recommendations can help ensure successful outcomes:
Getting Started with Retatrutide Research
Initial Planning Steps
- Literature Review: Conduct comprehensive review of existing retatrutide research and related triple agonist studies
- Feasibility Assessment: Evaluate whether your laboratory has necessary equipment, expertise, and resources
- Pilot Studies: Plan small-scale pilot experiments before committing to large studies
- Collaboration Exploration: Identify potential collaborators with complementary expertise
- Funding Considerations: Assess budget requirements and identify funding sources
Establishing Laboratory Capabilities
Essential Equipment
- Ultra-low temperature freezer (-80°C) for long-term storage
- Refrigeration (2-8°C) for reconstituted peptide
- Analytical balance for accurate measurements
- Sterile technique supplies and workspace
- Appropriate assay equipment for chosen endpoints
Technical Expertise
- Peptide handling and reconstitution skills
- Receptor-based assay experience
- Metabolic measurement capabilities
- Data analysis proficiency
- Quality control knowledge
Sourcing and Procurement
Selecting a Supplier
When choosing a supplier for LY3437943 retatrutide:
✅ Verify Quality Documentation
- Request sample Certificates of Analysis
- Confirm purity standards meet your requirements
- Check analytical methods used for quality control
- Verify batch-to-batch consistency data
✅ Evaluate Service Quality
- Test responsiveness to inquiries
- Assess technical knowledge of staff
- Review shipping and handling procedures
- Confirm return/replacement policies
✅ Consider Logistics
- Delivery timeframes to your location
- Temperature-controlled shipping options
- Customs/import procedures (for international orders)
- Minimum order quantities and pricing
PEPTIDE PRO offers researchers a comprehensive solution with high-purity retatrutide, fast UK delivery (same-day dispatch for orders before 1pm), international shipping options, and professional technical support.
Ordering Considerations
Quantity Planning
- Calculate total peptide needed for planned experiments
- Include buffer for optimization and troubleshooting
- Consider shelf-life and stability limitations
- Account for quality control testing requirements
- Plan for unexpected experimental needs
Budget Management
- Compare pricing across reputable suppliers
- Consider total cost including shipping
- Factor in potential waste from optimization
- Budget for related supplies (reconstitution solutions, assay reagents)
- Plan for follow-up studies
Optimizing Experimental Protocols
Protocol Development Process
- Start with Literature Protocols: Adapt published methods when available
- Optimize Systematically: Vary one parameter at a time
- Document Thoroughly: Record all conditions and outcomes
- Validate Rigorously: Confirm reproducibility before large studies
- Refine Continuously: Improve protocols based on experience
Common Optimization Parameters
- Peptide concentration range
- Incubation times and temperatures
- Cell density or tissue amount
- Buffer composition and pH
- Detection method sensitivity
- Control compound selection
Quality Assurance in Research
Maintaining Research Quality
Peptide Quality Control
- Verify appearance before each use
- Monitor storage conditions continuously
- Track reconstitution dates and stability
- Test new batches in parallel with previous batches
- Maintain detailed inventory records
Experimental Quality Control
- Include appropriate controls in every experiment
- Run positive controls to verify assay performance
- Monitor baseline variability
- Assess inter-assay reproducibility
- Implement standard operating procedures
Data Quality
- Establish data acceptance criteria
- Identify and address outliers appropriately
- Maintain raw data files
- Document all data transformations
- Implement version control for analyses
Troubleshooting Guide
| Issue | Possible Causes | Solutions |
|---|---|---|
| Poor reproducibility | Peptide degradation, inconsistent technique | Check storage conditions, standardize procedures, use fresh aliquots |
| Weak signal | Low peptide concentration, receptor expression issues | Optimize concentration, verify receptor presence, enhance detection |
| High background | Contamination, non-specific binding | Improve sterile technique, optimize blocking conditions, use fresh reagents |
| Variable dose-response | Peptide instability, pipetting errors | Prepare fresh dilutions, calibrate pipettes, use appropriate tips |
| Unexpected results | Off-target effects, model system issues | Include appropriate controls, validate model, review literature |
Collaboration and Knowledge Sharing
Building Research Networks
- Present findings at scientific conferences
- Publish methods and protocols
- Engage with online research communities
- Establish collaborations with complementary expertise
- Participate in multi-center studies when appropriate
Staying Current
- Monitor literature for new retatrutide research
- Follow developments in triple agonist field
- Track regulatory and safety updates
- Attend relevant scientific meetings
- Engage with supplier technical resources
Ethical Research Conduct
Responsible Research Practices
- Adhere to “For Research Use Only” designation
- Follow all institutional guidelines
- Maintain appropriate documentation
- Report findings accurately and completely
- Acknowledge limitations transparently
Safety and Compliance
- Follow laboratory safety protocols
- Use appropriate personal protective equipment
- Properly dispose of peptide waste
- Train all personnel adequately
- Maintain Material Safety Data Sheets (MSDS)
�
� Conclusion
LY3437943 retatrutide represents a significant advancement in metabolic research peptides, offering researchers an unprecedented tool for investigating multi-receptor metabolic regulation through its unique triple agonist mechanism targeting GIP, GLP-1, and glucagon receptors simultaneously. Throughout this comprehensive guide, we have explored the molecular characteristics, mechanisms of action, research applications, handling protocols, and practical considerations essential for successful retatrutide research in 2026.
Key Points Summary
Scientific Significance
- Retatrutide’s triple agonist profile enables comprehensive metabolic pathway investigation
- The compound provides unique insights into receptor cross-talk and synergistic effects
- Multi-pathway activation offers advantages over single or dual agonist approaches
- Research applications span fundamental receptor pharmacology to complex metabolic studies
Practical Research Considerations
- Proper handling, reconstitution, and storage are critical for maintaining peptide integrity
- High-purity research-grade material with comprehensive quality documentation is essential
- Rigorous experimental design with appropriate controls ensures reliable results
- Comparative studies with related compounds provide valuable mechanistic insights
Quality and Sourcing
- Research-grade retatrutide should meet ≥98% purity standards
- Certificates of Analysis provide essential quality documentation
- Reputable suppliers like PEPTIDE PRO offer high-quality peptides with professional service
- Proper supplier selection impacts research quality and reproducibility
Future Outlook
The research landscape for LY3437943 retatrutide continues to expand, with growing interest in:
- Advanced mechanistic understanding of triple receptor agonism
- Novel research applications beyond traditional metabolic studies
- Technological innovations enabling more sophisticated investigations
- Collaborative research approaches addressing complex questions
- Translational insights from fundamental research findings
As analytical technologies advance and research methodologies become more sophisticated, retatrutide will likely play an increasingly important role in metabolic research, systems pharmacology, and translational science.
Next Steps for Researchers
For scientists considering incorporating LY3437943 retatrutide into their research programs:
Immediate Actions
- Assess Research Fit: Determine whether retatrutide aligns with your specific research objectives and questions
- Evaluate Capabilities: Ensure your laboratory has necessary equipment, expertise, and resources
- Review Literature: Conduct comprehensive literature review of existing retatrutide research
- Plan Experiments: Develop detailed experimental protocols with appropriate controls
- Source Quality Peptide: Identify reputable supplier offering research-grade retatrutide with proper documentation
Getting Started
- Explore PEPTIDE PRO’s research peptide catalogue to view available compounds including retatrutide
- Contact PEPTIDE PRO’s technical team for product information, COAs, and research support
- Review handling and storage protocols to ensure proper peptide management
- Design pilot studies to optimize protocols before large-scale experiments
- Establish quality control procedures for ongoing research
Building Research Program
- Develop collaborations with complementary expertise
- Stay current with emerging retatrutide research
- Implement rigorous quality assurance practices
- Document protocols and findings thoroughly
- Share knowledge with scientific community
Final Thoughts
LY3437943 retatrutide exemplifies the evolution of metabolic research peptides toward more comprehensive, multi-pathway approaches. Its triple agonist mechanism provides researchers with a powerful tool for investigating complex metabolic regulation, receptor interactions, and integrated physiological responses. Success with retatrutide research requires attention to quality, rigorous experimental design, proper handling procedures, and commitment to scientific excellence.
The research community’s growing understanding of multi-receptor agonism, combined with advancing technologies and collaborative approaches, positions retatrutide research at the forefront of metabolic science in 2026. Researchers working with this compound contribute to fundamental knowledge that may ultimately inform therapeutic strategies, deepen understanding of metabolic regulation, and advance translational medicine.
For laboratories seeking to advance their metabolic research programs with high-quality research peptides, PEPTIDE PRO stands ready to support your scientific endeavors with premium research-grade LY3437943 retatrutide, comprehensive quality documentation, fast delivery, and professional service trusted by researchers across the UK and worldwide.
Research Commitment: All peptides supplied by PEPTIDE PRO are clearly labeled “For Research Use Only” and are not intended for human or animal consumption. Researchers must adhere to appropriate institutional guidelines, ethical standards, and safety protocols when conducting peptide research.
