The landscape of weight management treatment has transformed dramatically with the introduction of tirzepatide, a groundbreaking medication that’s reshaping how the NHS approaches obesity care. Understanding tirzepatide NHS eligibility BMI requirements has become essential for millions of people seeking effective weight loss solutions through the health service. With obesity rates continuing to climb and new treatment options emerging, navigating the complex eligibility criteria can feel overwhelming—but knowing the specific BMI thresholds and additional requirements could be your first step toward accessing this potentially life-changing therapy.
As healthcare systems worldwide grapple with the obesity epidemic, tirzepatide represents a significant advancement in pharmacological weight management. However, access through the NHS isn’t automatic, and the eligibility framework centres heavily on body mass index (BMI) measurements alongside other clinical factors. Whether you’re a healthcare professional seeking clarity on prescribing guidelines or an individual exploring treatment options, this comprehensive guide will demystify the tirzepatide NHS eligibility BMI criteria and help you understand the complete pathway to access.
Key Takeaways
- BMI threshold of 30 kg/m² or higher is typically required for tirzepatide NHS eligibility, with lower thresholds (27 kg/m²) possible for patients with weight-related comorbidities
- Type 2 diabetes diagnosis is currently a primary requirement for NHS tirzepatide prescribing, though this may evolve as NICE guidance develops
- Tier 3 weight management services often serve as gatekeepers, requiring patients to complete structured lifestyle interventions before medication access
- Postcode lottery concerns persist, with significant regional variation in tirzepatide availability across different NHS trusts and ICBs
- Research-grade tirzepatide remains available through specialized suppliers like PEPTIDE PRO for scientific investigation purposes only
Understanding Tirzepatide: Mechanism and Clinical Significance
Tirzepatide represents a novel class of medication known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. This dual-action mechanism sets it apart from earlier weight management medications, including semaglutide, by targeting two complementary metabolic pathways simultaneously.
How Tirzepatide Works
The medication functions by:
- Enhancing insulin secretion in response to food intake, improving glucose control
- Suppressing glucagon release when blood sugar levels are elevated
- Slowing gastric emptying, which promotes satiety and reduces appetite
- Acting on brain appetite centers to decrease food cravings and portion sizes
- Improving insulin sensitivity in peripheral tissues
Clinical trials have demonstrated remarkable efficacy, with participants achieving average weight reductions of 15-22% of their initial body weight over 72 weeks—significantly outperforming previous pharmaceutical interventions. These results have generated substantial interest from both medical professionals and patients seeking effective obesity management solutions.
Clinical Trial Evidence
The SURMOUNT clinical trial programme has provided robust evidence for tirzepatide’s effectiveness:
| Trial | Population | Average Weight Loss | Duration |
|---|---|---|---|
| SURMOUNT-1 | Adults with obesity (no diabetes) | 20.9% (15mg dose) | 72 weeks |
| SURMOUNT-2 | Adults with obesity and type 2 diabetes | 15.7% (15mg dose) | 72 weeks |
| SURMOUNT-3 | Obesity with intensive lifestyle intervention | 18.4% maintained | 72 weeks |
| SURMOUNT-4 | Weight maintenance after initial loss | Additional 5.5% loss | 52 weeks |
These impressive outcomes have accelerated regulatory approvals and sparked discussions about expanding NHS access beyond current restrictions.
Tirzepatide NHS Eligibility BMI: Primary Requirements
The cornerstone of tirzepatide NHS eligibility BMI criteria revolves around specific body mass index thresholds, though these exist within a broader clinical framework that considers multiple factors.
Standard BMI Thresholds
For most NHS patients, tirzepatide eligibility typically requires:
Primary threshold: BMI ≥ 30 kg/m² (classified as obese)
This aligns with standard obesity definitions used throughout NHS weight management services. However, the eligibility framework recognizes that BMI alone doesn’t capture the full clinical picture, particularly for certain ethnic groups and individuals with specific health conditions.
Adjusted BMI Criteria for Ethnic Minorities
The NHS acknowledges that obesity-related health risks occur at lower BMI levels in certain populations. Adjusted thresholds apply for individuals of:
- South Asian descent: BMI ≥ 27.5 kg/m²
- Chinese, Black African, or African-Caribbean descent: BMI ≥ 28 kg/m²
- Other non-white ethnic groups: Individual clinical assessment
These adjustments reflect evidence that metabolic complications and cardiovascular risks emerge at lower BMI levels in these populations, making earlier intervention clinically appropriate.
Lower BMI Threshold with Comorbidities
Patients with a BMI of 27-29.9 kg/m² (overweight but not obese) may qualify for tirzepatide if they have:
✅ Type 2 diabetes (currently the primary qualifying condition)
✅ Hypertension requiring medication
✅ Dyslipidemia (abnormal cholesterol levels)
✅ Obstructive sleep apnea
✅ Non-alcoholic fatty liver disease (NAFLD)
✅ Cardiovascular disease
✅ Polycystic ovary syndrome (PCOS)
The presence of these weight-related comorbidities demonstrates that excess weight is already causing health complications, justifying pharmaceutical intervention at a lower BMI threshold.
Type 2 Diabetes: The Current Gateway Criterion
As of 2026, the most significant factor determining tirzepatide NHS eligibility BMI access is a confirmed diagnosis of type 2 diabetes. This requirement stems from tirzepatide’s initial licensing and NICE guidance, which prioritized the medication for patients who would benefit from both its weight loss and glycemic control properties.
Why Diabetes Matters for NHS Access
Tirzepatide received UK regulatory approval initially as a diabetes medication (marketed as Mounjaro), with weight loss recognized as a substantial beneficial side effect. The NHS typically follows NICE (National Institute for Health and Care Excellence) guidance, which has approved tirzepatide specifically for:
“Adults with type 2 diabetes mellitus and a body mass index of at least 30 kg/m² (or 27 kg/m² with weight-related comorbidities) when diet and exercise alone have not provided adequate glycemic control.”
This diabetes-centric approach means that individuals seeking tirzepatide purely for weight management—without a diabetes diagnosis—face significant barriers to NHS access, regardless of their BMI.
Glycemic Control Requirements
Beyond simply having a diabetes diagnosis, many NHS trusts require evidence of:
- HbA1c levels above target range (typically >58 mmol/mol or 7.5%) despite other interventions
- Inadequate response to metformin or other first-line diabetes medications
- Documented lifestyle modification attempts including dietary changes and increased physical activity
- Diabetes duration of at least 6-12 months with consistent management efforts
These additional criteria ensure that tirzepatide is reserved for patients who genuinely need advanced therapeutic options rather than serving as a first-line treatment.
The Weight-Only Indication Gap
While tirzepatide has received FDA approval in the United States for chronic weight management in adults without diabetes (marketed as Zepbound), UK regulatory and NICE approval for this indication has been slower to materialize. This creates a significant access gap for the estimated 26% of UK adults with obesity who don’t have diabetes but could benefit substantially from the medication’s weight loss effects.
Advocacy groups continue pressing for expanded NICE guidance that would enable NHS prescribing for weight management independent of diabetes status, particularly given the medication’s superior efficacy compared to previously approved weight loss drugs.
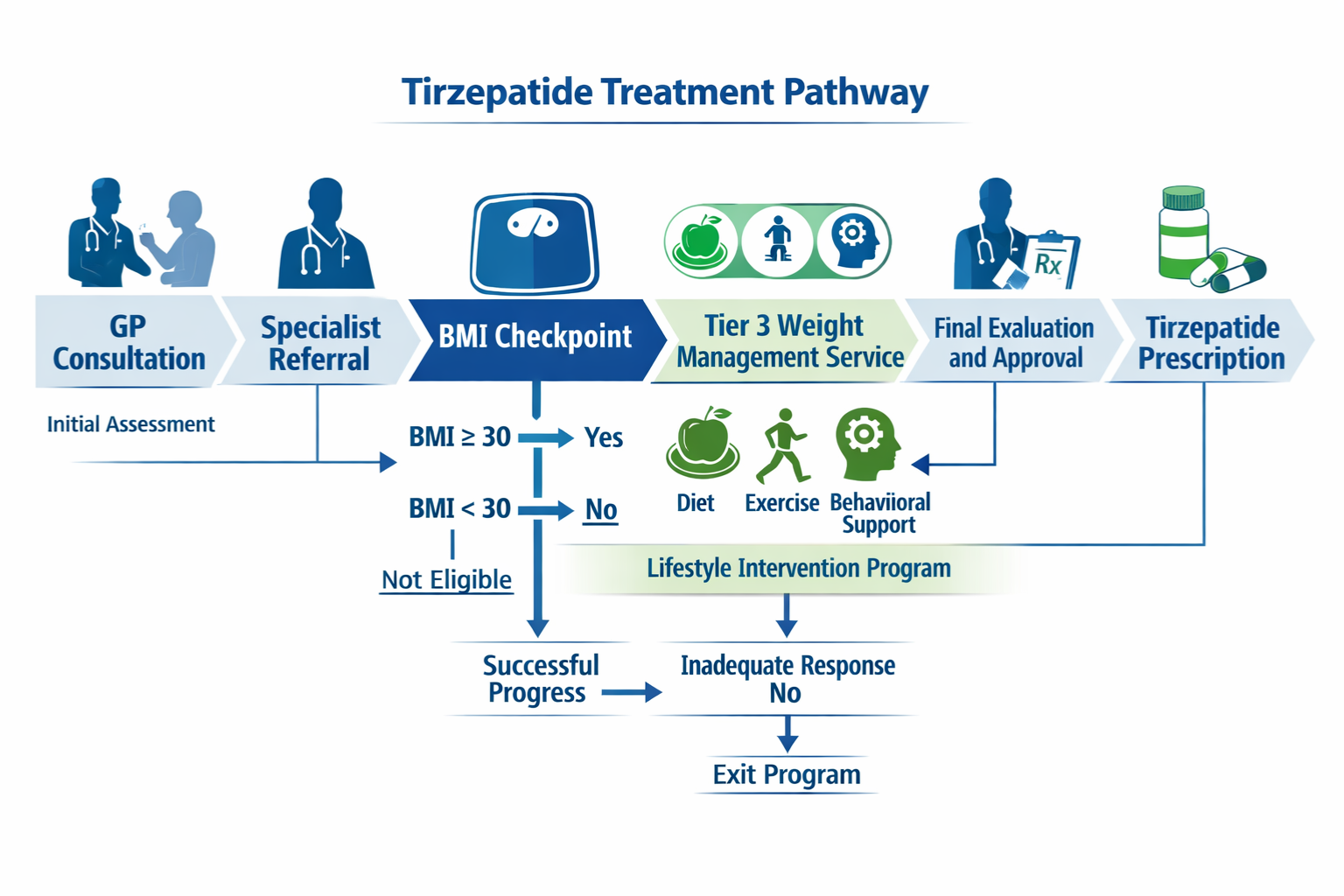
NHS Pathway to Tirzepatide Access: Step-by-Step Process
Understanding the tirzepatide NHS eligibility BMI criteria is only the first step. Actually accessing the medication through the NHS involves navigating a structured pathway with multiple gatekeepers and requirements.
Step 1: Primary Care Assessment
The journey typically begins with your GP, who will:
- Calculate your BMI using current height and weight measurements
- Review your medical history for type 2 diabetes and weight-related comorbidities
- Assess previous weight management attempts, including diet modifications, exercise programmes, and any previous medications
- Conduct baseline blood tests including HbA1c, lipid profile, liver function, and kidney function
- Screen for contraindications such as personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2
If you meet the basic criteria, your GP may refer you to specialist weight management services rather than prescribing directly.
Step 2: Tier 3 Weight Management Services
Most NHS regions require patients to complete a Tier 3 weight management programme before accessing medications like tirzepatide. These multidisciplinary services typically involve:
- Dietitian consultations to develop personalized nutrition plans
- Physical activity specialists who create tailored exercise programmes
- Psychological support addressing emotional eating and behavioral change
- Medical monitoring of weight, blood pressure, and metabolic markers
- Programme duration of 6-12 months with regular appointments
The rationale is that lifestyle interventions should be optimized before adding pharmaceutical treatments, and that medication works best when combined with sustained behavioral changes.
⚠️ Important: Tier 3 service availability varies dramatically by region, with some areas having waiting lists exceeding 12 months. This represents a significant access barrier even for eligible patients.
Step 3: Specialist Prescribing Decision
Following Tier 3 assessment, a specialist (typically an endocrinologist, diabetologist, or specialist weight management physician) will:
- Review your complete medical history and Tier 3 programme outcomes
- Confirm tirzepatide eligibility against local ICB (Integrated Care Board) criteria
- Discuss treatment expectations, including realistic weight loss goals and potential side effects
- Obtain informed consent after explaining risks, benefits, and alternatives
- Initiate prescribing if all criteria are met and local funding is available
Step 4: Ongoing Monitoring and Continuation Criteria
NHS tirzepatide prescribing isn’t indefinite. Continuation typically requires:
📊 3-month review: Minimum 5% weight loss from baseline
📊 6-month review: Minimum 10% weight loss or significant HbA1c improvement
📊 Ongoing assessments: Continued weight maintenance and tolerability
📊 Maximum duration: Often limited to 2 years, though this varies by ICB
Failure to achieve these milestones typically results in treatment discontinuation, as the medication is considered ineffective for that individual.
Regional Variation: The Tirzepatide Postcode Lottery
One of the most frustrating aspects of tirzepatide NHS eligibility BMI access is the significant geographical variation in availability. Despite national NICE guidance, individual Integrated Care Boards (ICBs) retain considerable autonomy in funding decisions, creating what critics call a “postcode lottery.”
ICB Funding Decisions
Each of England’s 42 ICBs makes independent decisions about:
- Whether to fund tirzepatide at all within their area
- Additional eligibility restrictions beyond NICE guidance
- Prescribing volume limits and budget caps
- Preferred treatment pathways and mandatory prerequisites
- Tier 3 service capacity and referral criteria
As of 2026, some ICBs have embraced tirzepatide prescribing with relatively open access for eligible patients, while others have imposed strict limitations or effectively blocked access through restrictive local policies.
Examples of Regional Differences
| Region | Tirzepatide Access Status | Notable Restrictions |
|---|---|---|
| London (various ICBs) | Variable | Some ICBs require 12-month Tier 3 completion; others have suspended new initiations due to budget pressures |
| Manchester | Moderate access | BMI ≥35 required unless multiple comorbidities present |
| Birmingham & Solihull | Limited | Restricted to specialist diabetes clinics only |
| Cornwall & Isles of Scilly | Good access | Follows NICE guidance with 6-month Tier 3 requirement |
| Norfolk & Waveney | Very limited | Effective moratorium on new prescriptions since late 2025 |
These disparities mean that two patients with identical clinical profiles may receive completely different answers about tirzepatide access depending solely on where they live.
Advocacy and Policy Challenges
Patient advocacy organizations have increasingly challenged this variation, arguing that:
- Postcode lotteries violate NHS principles of equitable access based on clinical need
- Budget constraints shouldn’t override clinical evidence when medications demonstrate clear benefits
- Obesity should be treated as seriously as other chronic conditions with consistent national access
- Short-term cost concerns ignore long-term savings from preventing obesity-related complications
The debate continues, with some calling for mandatory national implementation of NICE-approved treatments to eliminate regional variation.
Alternative Pathways and Private Access
Given the challenges of NHS access, many patients explore alternative routes to tirzepatide, though each comes with important considerations.
Private Prescription Options
Patients who meet clinical criteria but face NHS barriers may access tirzepatide through:
Private endocrinologists and weight management clinics
- Typical costs: £150-300 per month for medication plus consultation fees
- Faster access without Tier 3 programme requirements
- More flexible eligibility criteria
- Ongoing monitoring and support included
Online prescription services
- Convenient remote consultations
- Variable quality and clinical oversight
- Prices often competitive with traditional private clinics
- Important to verify GMC registration and CQC ratings
Research-Grade Peptides
For scientific research purposes only, organizations like PEPTIDE PRO supply high-purity research-grade peptides including tirzepatide preparations. These products are:
⚠️ Strictly for research use only – not for human consumption
⚠️ Intended for laboratory investigation and scientific study
⚠️ Supplied with certificates of analysis confirming purity and composition
⚠️ Subject to proper handling and storage requirements
Researchers investigating metabolic pathways, peptide mechanisms, or related scientific questions can access these materials through proper channels. PEPTIDE PRO maintains rigorous quality standards and provides comprehensive support for legitimate research applications.
Medical Tourism Considerations
Some patients consider accessing tirzepatide through:
- European clinics where prescribing criteria may differ
- Medical tourism destinations offering weight management packages
- International online pharmacies (with significant safety and legality concerns)
Critical warnings: ❌ Unregulated sources may supply counterfeit or contaminated products
❌ Lack of medical supervision increases safety risks
❌ Importing prescription medications may violate UK law
❌ Insurance and legal protections don’t apply to treatments obtained abroad
Beyond BMI: Holistic Eligibility Considerations
While tirzepatide NHS eligibility BMI forms the foundation of access criteria, comprehensive assessment considers numerous additional factors that may support or preclude treatment.
Supporting Factors That Strengthen Eligibility
Healthcare providers consider:
Weight-related quality of life impairment
- Mobility limitations affecting daily activities
- Inability to work or reduced work capacity
- Social isolation and mental health impacts
- Failed previous weight loss attempts despite genuine effort
Obesity-related complications
- Joint problems requiring or likely to require surgery
- Respiratory difficulties beyond sleep apnea
- Fertility issues related to weight
- Skin conditions from excess weight
Psychological readiness
- Realistic expectations about treatment outcomes
- Commitment to lifestyle modifications alongside medication
- Understanding of potential side effects and management strategies
- Support systems in place for long-term change
Absolute Contraindications
Certain conditions preclude tirzepatide use regardless of BMI:
🚫 Personal or family history of medullary thyroid carcinoma
🚫 Multiple endocrine neoplasia syndrome type 2 (MEN 2)
🚫 Pregnancy or breastfeeding (category C medication)
🚫 Severe gastroparesis or gastrointestinal disorders
🚫 History of pancreatitis (relative contraindication requiring specialist assessment)
🚫 Severe renal impairment (eGFR <30 mL/min/1.73m²)
Relative Contraindications Requiring Careful Assessment
Additional factors that may complicate prescribing decisions:
- Active gallbladder disease (tirzepatide may increase gallstone risk)
- History of diabetic retinopathy (rapid glucose lowering may temporarily worsen retinopathy)
- Concurrent use of insulin or sulfonylureas (increased hypoglycemia risk)
- Eating disorders history (medication affecting appetite requires psychological assessment)
- Age considerations (limited data in patients over 75 or under 18)
Preparing for Your Tirzepatide Eligibility Assessment
If you’re considering pursuing tirzepatide through the NHS, thorough preparation can strengthen your case and streamline the process.
Documentation to Gather
📋 Weight history records
- Historical BMI measurements from GP records
- Documentation of previous weight loss attempts
- Records of any commercial programmes (Slimming World, Weight Watchers, etc.)
- Food diaries or activity logs if available
📋 Medical history compilation
- Complete list of current medications
- Documentation of diabetes diagnosis and management
- Records of weight-related complications
- Family history of obesity and related conditions
📋 Lifestyle modification evidence
- Proof of gym membership or exercise programme participation
- Dietitian consultation records
- Evidence of dietary changes implemented
- Behavioral modification programme completion
Questions to Ask Your Healthcare Provider
When discussing tirzepatide NHS eligibility BMI with your GP or specialist:
- “Do I meet the BMI threshold for tirzepatide in our ICB?”
- “What additional criteria must I fulfill beyond BMI and diabetes?”
- “Is Tier 3 weight management service completion mandatory in our area?”
- “What is the current waiting time for Tier 3 services?”
- “Are there funding restrictions or prescribing limits currently in place?”
- “What weight loss milestones will be required to continue treatment?”
- “What are the most common side effects, and how are they managed?”
- “What happens if I don’t achieve the required weight loss targets?”
Setting Realistic Expectations
Understanding what tirzepatide can and cannot achieve helps maintain appropriate expectations:
Realistic outcomes:
- Average weight loss of 15-20% over 12-18 months when combined with lifestyle changes
- Significant improvements in blood sugar control for diabetic patients
- Reductions in blood pressure and cholesterol levels
- Enhanced quality of life and physical function
Common misconceptions to avoid:
- Tirzepatide is not a “quick fix” requiring no effort
- Weight loss occurs gradually, not overnight
- Side effects (especially nausea) are common initially
- Lifestyle modifications remain essential for success
- Weight regain is possible after discontinuation without maintained habits
The Future of Tirzepatide NHS Access
The landscape of tirzepatide NHS eligibility BMI criteria continues to evolve as new evidence emerges and healthcare policies adapt.
Anticipated NICE Guidance Updates
Several developments may expand access:
Weight management indication approval
- NICE is currently reviewing tirzepatide for obesity treatment independent of diabetes
- Approval expected potentially in late 2026 or early 2027
- Would significantly broaden eligible patient population
- May still require high BMI thresholds (≥35 or ≥30 with comorbidities)
Cardiovascular outcomes data
- Ongoing trials examining heart disease prevention benefits
- Positive results could justify earlier intervention at lower BMI levels
- May influence cost-effectiveness assessments favoring broader access
Real-world effectiveness evidence
- NHS pilot programmes collecting data on implementation outcomes
- Evidence of sustained weight loss and comorbidity improvement strengthens case for funding
- Demonstration of cost savings through reduced complications supports expansion
Supply Chain and Manufacturing Considerations
Current challenges affecting access include:
- Global supply constraints due to unprecedented demand
- Manufacturing scale-up by Eli Lilly to meet worldwide needs
- Prioritization decisions about which markets receive available supply
- Potential biosimilar development in coming years as patents approach expiration
These practical considerations may temporarily restrict access even where clinical eligibility is met.
Policy Advocacy Opportunities
Patients and healthcare professionals can support expanded access through:
- Engaging with local ICBs during public consultation periods
- Participating in patient advocacy organizations focused on obesity treatment
- Sharing experiences with MPs and health policy makers
- Contributing to research and real-world evidence collection
- Challenging restrictive policies through appropriate channels
Complementary Approaches to Weight Management
Regardless of tirzepatide NHS eligibility BMI status, comprehensive weight management requires multifaceted approaches.
Evidence-Based Lifestyle Interventions
Dietary modifications:
- Mediterranean diet patterns showing consistent benefits
- Portion control strategies and mindful eating practices
- Reducing ultra-processed food consumption
- Adequate protein intake to preserve muscle mass during weight loss
Physical activity:
- Minimum 150 minutes moderate-intensity activity weekly
- Resistance training to maintain metabolic rate
- Increasing non-exercise activity thermogenesis (NEAT)
- Finding sustainable, enjoyable activities rather than punishment-based exercise
Behavioral strategies:
- Cognitive behavioral therapy for eating behaviors
- Stress management and sleep optimization
- Social support networks and accountability
- Environmental modifications to support healthy choices
Other Pharmacological Options
If tirzepatide access proves impossible, alternative medications include:
| Medication | Mechanism | Average Weight Loss | NHS Availability |
|---|---|---|---|
| Semaglutide (Wegovy) | GLP-1 agonist | 12-15% | Limited NHS access, similar restrictions to tirzepatide |
| Orlistat | Lipase inhibitor | 3-5% | Widely available on NHS, lower efficacy |
| Naltrexone-bupropion | Appetite suppressant | 5-8% | Not routinely funded by NHS |
| Liraglutide (Saxenda) | GLP-1 agonist | 5-8% | Very limited NHS access |
For research purposes, PEPTIDE PRO supplies various research-grade peptides for scientific investigation, maintaining strict quality standards and proper documentation for legitimate research applications.
Surgical Interventions
For patients with BMI ≥40 (or ≥35 with comorbidities), bariatric surgery remains an option:
Gastric bypass
- Average 25-30% total body weight loss
- Significant metabolic benefits
- More invasive with longer recovery
Sleeve gastrectomy
- Average 20-25% total body weight loss
- Fewer complications than bypass
- Increasingly common NHS option
Gastric band
- Variable outcomes, less commonly performed
- Reversible but requires ongoing adjustments
- Lower complication rates but also lower efficacy
NHS bariatric surgery also requires completion of Tier 3 services and demonstration of lifestyle modification commitment.
Conclusion: Navigating Tirzepatide NHS Eligibility in 2026
Understanding tirzepatide NHS eligibility BMI requirements represents just one piece of a complex healthcare puzzle. While the medication offers unprecedented effectiveness for weight management and metabolic health, accessing it through the NHS requires meeting multiple criteria, navigating regional variations, and often demonstrating commitment through structured weight management programmes.
The current landscape places tirzepatide primarily within diabetes treatment pathways, with BMI thresholds of 30 kg/m² (or 27 kg/m² with comorbidities) serving as the foundation for eligibility. However, the reality of access extends far beyond these numbers, incorporating diabetes diagnosis requirements, Tier 3 service completion, regional funding decisions, and ongoing monitoring criteria.
Key Action Steps
If you’re considering pursuing tirzepatide through the NHS:
- Calculate your BMI accurately and understand whether you meet the threshold for your ethnic background
- Document your weight management journey including all previous attempts and their outcomes
- Discuss options with your GP to understand local pathways and current availability
- Engage with Tier 3 services if required in your area, viewing them as valuable support rather than obstacles
- Stay informed about policy changes as NICE guidance evolves and access potentially expands
- Consider all treatment options including lifestyle interventions, alternative medications, and surgical approaches
- Explore legitimate alternatives if NHS access proves impossible, while avoiding unregulated sources
Looking Ahead
The trajectory of tirzepatide access appears positive, with potential expansion of NICE guidance, increased manufacturing capacity, and growing recognition of obesity as a chronic disease requiring effective pharmaceutical interventions. However, change in healthcare policy occurs gradually, and current access barriers will likely persist for the immediate future.
For those engaged in legitimate scientific research, PEPTIDE PRO continues to provide high-purity research-grade peptides with comprehensive quality documentation, supporting the advancement of metabolic science and peptide research.
Ultimately, whether you access tirzepatide through the NHS, private channels, or pursue alternative approaches, success in weight management requires commitment to sustainable lifestyle changes, appropriate medical supervision, and realistic expectations about outcomes. The medication, when available, serves as a powerful tool—but remains just one component of comprehensive, long-term health optimization.
