The NHS rollout of tirzepatide (Mounjaro) represents one of the most significant developments in obesity management in recent years—but accessing this groundbreaking medication isn’t straightforward. With strict tirzepatide prescription UK criteria governing who qualifies, understanding the eligibility requirements has never been more critical for patients and healthcare professionals alike. As of 2026, the phased implementation continues to evolve, creating confusion about BMI thresholds, qualifying conditions, and the comprehensive assessment process required before treatment can begin.
This professional guide demystifies the tirzepatide prescription UK criteria, providing clear, evidence-based information about eligibility requirements, the application process, safety considerations, and what patients can expect from the NHS pathway. Whether you’re a healthcare professional navigating the Local Enhanced Service framework or a patient exploring your treatment options, this resource delivers the authoritative answers you need.
Key Takeaways
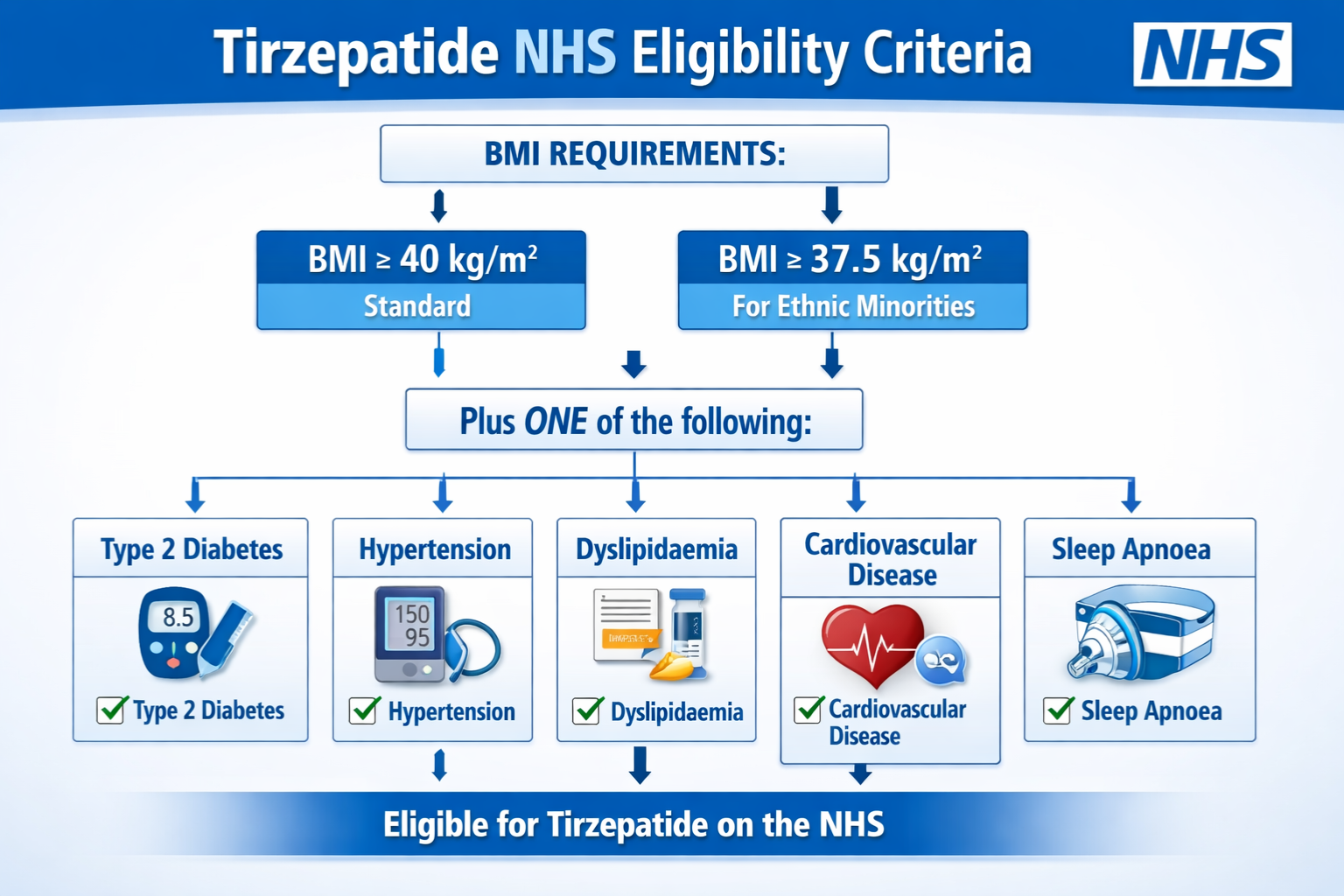
- BMI thresholds vary by ethnicity: Standard eligibility requires BMI ≥40 kg/m², or ≥37.5 kg/m² for South Asian, Chinese, other Asian, Middle Eastern, Black African, or African-Caribbean backgrounds
- Multiple qualifying conditions required: Patients must have at least 4 of 5 specific health conditions (Type 2 Diabetes, Hypertension, Dyslipidaemia, Cardiovascular Disease, or Obstructive Sleep Apnoea)
- Phased rollout continues through 2027: Three cohorts determine access, with Cohort 2 (BMI 35-39.9) starting June 2026 and Cohort 3 (BMI ≥40 with 3 conditions) beginning April 2027
- Mandatory behavioural support: All NHS tirzepatide prescriptions require engagement with the Behavioural Support for Obesity Programme (BSOP)
- Strict continuation criteria apply: Patients must achieve minimum 5% weight loss at 6 months to continue treatment
Understanding Tirzepatide and Its Role in Obesity Management
Tirzepatide represents a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, offering a novel mechanism of action that distinguishes it from previous weight management medications. Originally developed for Type 2 Diabetes management, clinical trials demonstrated remarkable weight loss outcomes, prompting regulatory approval for obesity treatment.
The medication works by mimicking natural hormones that regulate appetite, glucose metabolism, and energy expenditure. Clinical trials showed average weight loss of 15-20% of body weight, significantly exceeding results from earlier GLP-1 medications. This efficacy prompted NHS England to prioritize tirzepatide access despite substantial budgetary implications.
Why NHS England Implemented Strict Criteria
The decision to establish rigorous tirzepatide prescription UK criteria stems from multiple factors:
💷 Financial Sustainability: With treatment costs exceeding £200 per patient monthly and an estimated 3.4 million potentially eligible patients across England, unrestricted access would create unsustainable NHS expenditure. The phased approach allows controlled budget allocation while maximizing clinical benefit for those at highest risk.
📊 Clinical Prioritization: Evidence demonstrates greatest benefit for patients with multiple obesity-related complications. By requiring multiple qualifying conditions, the NHS ensures treatment reaches those facing most significant health risks and potential complications.
🔬 Long-term Safety Monitoring: The gradual rollout enables comprehensive safety surveillance, adverse event tracking, and real-world effectiveness data collection across diverse patient populations.
🏥 Healthcare Infrastructure: GP practices require time to implement the Obesity Local Enhanced Service framework, train staff, establish monitoring protocols, and integrate behavioural support programmes.
For researchers and institutions exploring peptide compounds for scientific investigation, PEPTIDE PRO provides research-grade materials with comprehensive documentation and quality assurance.
Complete Tirzepatide Prescription UK Criteria for 2026
Understanding the precise eligibility requirements is essential for both patients and prescribers. The tirzepatide prescription UK criteria encompass multiple domains, each requiring careful assessment and documentation.
BMI Requirements: Ethnicity-Adjusted Thresholds
Body Mass Index forms the foundational eligibility criterion, with important adjustments recognizing ethnic variations in obesity-related health risks:
Standard BMI Threshold:
- ≥40 kg/m² for patients of White European, Mixed, or Other ethnic backgrounds
Adjusted BMI Threshold:
- ≥37.5 kg/m² for patients from:
- South Asian backgrounds (Indian, Pakistani, Bangladeshi, Sri Lankan)
- Chinese backgrounds
- Other Asian backgrounds
- Middle Eastern backgrounds
- Black African backgrounds
- African-Caribbean backgrounds
These adjusted thresholds reflect robust epidemiological evidence demonstrating earlier onset and greater severity of obesity-related complications in these populations at lower BMI levels.
The Five Qualifying Health Conditions
Beyond BMI requirements, patients must have at least 4 of the following 5 qualifying conditions:
| Qualifying Condition | Diagnostic Criteria | Documentation Required |
|---|---|---|
| Type 2 Diabetes | HbA1c ≥48 mmol/mol or confirmed diagnosis with current treatment | Recent HbA1c result, medication history |
| Hypertension | BP ≥140/90 mmHg or confirmed diagnosis with antihypertensive treatment | Multiple BP readings, current medications |
| Dyslipidaemia | Abnormal lipid profile or current lipid-lowering therapy | Recent lipid panel (total cholesterol, LDL, HDL, triglycerides) |
| Cardiovascular Disease | Documented history of MI, stroke, angina, peripheral arterial disease, or heart failure | Medical records, diagnostic imaging, specialist letters |
| Obstructive Sleep Apnoea | Confirmed diagnosis via sleep study or clinical assessment | Sleep study results, CPAP usage records, specialist confirmation |
This multi-morbidity requirement ensures treatment targets patients experiencing greatest health burden from obesity, where weight reduction delivers maximum clinical benefit across multiple systems.
Age, Pregnancy, and Contraindication Criteria
Age Restrictions:
- ✅ Patients must be 18 years or older
- ❌ Tirzepatide is not available on NHS for patients under 18
Pregnancy and Breastfeeding:
- ❌ Absolute contraindication during pregnancy
- ❌ Not recommended while breastfeeding
- ⚠️ Females of childbearing potential must use effective contraception
- ⚠️ Treatment should be discontinued 2 months before planned pregnancy
Medical Contraindications:
- ❌ Personal history of medullary thyroid carcinoma (MTC)
- ❌ Family history of medullary thyroid carcinoma
- ❌ Multiple Endocrine Neoplasia syndrome type 2 (MEN2)
- ⚠️ Severe gastrointestinal disease (relative contraindication requiring specialist assessment)
- ⚠️ History of pancreatitis (requires careful risk-benefit evaluation)
- ⚠️ Severe renal impairment (requires dose adjustment and monitoring)
The Three-Cohort Phased Rollout System
NHS England structured tirzepatide access through three sequential cohorts, each with specific eligibility windows:
Cohort 1 (Started June 2025):
- BMI ≥40 kg/m² (or ≥37.5 kg/m² for eligible ethnic groups)
- At least 4 of 5 qualifying conditions
- Highest priority group
- Estimated 220,000 patients
Cohort 2 (Starting June 2026):
- BMI 35-39.9 kg/m² (or 32.5-37.4 kg/m² for eligible ethnic groups)
- At least 4 of 5 qualifying conditions
- Expansion to moderately severe obesity with high comorbidity burden
- Estimated additional 500,000 patients
Cohort 3 (Starting April 2027):
- BMI ≥40 kg/m² (or ≥37.5 kg/m² for eligible ethnic groups)
- At least 3 of 5 qualifying conditions (reduced threshold)
- Broadened access for severe obesity
- Estimated additional 800,000 patients
NHS England projects it will take up to 12 years for all eligible patients to access treatment due to capacity constraints and budgetary limitations.
The NHS Prescription Pathway: From Assessment to Treatment
Navigating the tirzepatide prescription UK criteria requires understanding the comprehensive assessment and initiation process established by NHS England.
GP Practice Registration and Local Enhanced Service
Only GP practices signed up to the Obesity Local Enhanced Service can prescribe tirzepatide for weight management. This framework ensures:
- 🏥 Practices have appropriate infrastructure and staffing
- 📋 Standardized assessment protocols are followed
- 📊 Comprehensive data collection for monitoring and evaluation
- 🤝 Integration with behavioural support services
- 💊 Appropriate medication management and monitoring systems
Patients should verify their GP practice participates in the scheme before requesting assessment. Non-participating practices cannot prescribe tirzepatide through the NHS pathway, regardless of patient eligibility.
Mandatory Baseline Assessments
Before tirzepatide initiation, comprehensive baseline assessments must be completed and documented:
Physical Measurements:
- ✓ Weight (kg) and height (m) for BMI calculation
- ✓ Waist circumference measurement
- ✓ Blood pressure (multiple readings)
- ✓ Resting heart rate
Laboratory Investigations:
- ✓ HbA1c (glycated haemoglobin)
- ✓ Fasting lipid profile (total cholesterol, LDL, HDL, triglycerides)
- ✓ Renal function (eGFR, creatinine)
- ✓ Liver function tests
- ✓ Thyroid function (if clinically indicated)
Clinical Documentation:
- ✓ Complete medical history
- ✓ Current medications and allergies
- ✓ Previous weight management attempts
- ✓ Smoking status and alcohol consumption
- ✓ Mental health screening
- ✓ Quality of life assessment
This comprehensive baseline establishes the foundation for monitoring treatment response and identifying potential safety concerns.
Behavioural Support for Obesity Programme (BSOP) Requirement
Engagement with BSOP wraparound care is mandatory for all NHS tirzepatide prescriptions. This requirement reflects evidence that combining pharmacotherapy with behavioural intervention produces superior long-term outcomes compared to medication alone.
BSOP components typically include:
Nutritional Counselling:
- Individualized dietary assessment and planning
- Education about portion control and food choices
- Strategies for managing emotional eating
- Practical meal planning and preparation guidance
Physical Activity Support:
- Personalized exercise recommendations based on current fitness
- Progressive activity goals with regular review
- Strategies for incorporating movement into daily routines
- Access to community exercise programmes where available
Behavioural Modification:
- Goal-setting and action planning techniques
- Self-monitoring strategies (food diaries, activity tracking)
- Cognitive-behavioural approaches to eating behaviours
- Stress management and emotional regulation skills
Ongoing Support:
- Regular group or individual sessions (frequency varies by programme)
- Peer support opportunities
- Digital resources and mobile applications
- Access to specialist obesity services when needed
Failure to engage with BSOP may result in treatment discontinuation, as the integrated approach forms a core component of the NHS obesity management pathway.
For research institutions investigating peptide-based interventions and requiring high-purity compounds for laboratory studies, PEPTIDE PRO’s research catalogue offers extensively characterized materials with comprehensive documentation.
Patient Counselling and Informed Consent
Before prescribing tirzepatide, comprehensive patient counselling must address:
Treatment Expectations:
- Realistic weight loss goals (typically 15-20% body weight over 12-18 months)
- Timeline for results (gradual weight loss, not immediate)
- Need for long-term commitment and lifestyle modification
- Potential for weight regain if treatment discontinued
Common Adverse Effects:
- Gastrointestinal symptoms (nausea, vomiting, diarrhoea, constipation)
- Injection site reactions
- Fatigue and decreased appetite
- Potential for gallbladder complications
- Risk of hypoglycaemia (especially with concurrent diabetes medications)
Safety Monitoring Requirements:
- Monthly appointments during titration
- Regular weight and vital sign monitoring
- Periodic laboratory testing
- Importance of reporting adverse effects promptly
Pregnancy Planning:
- Requirement for effective contraception in females of childbearing potential
- Need to discontinue 2 months before planned conception
- Risks of medication exposure during pregnancy
Lifestyle Commitment:
- Ongoing dietary modifications
- Regular physical activity
- Continued BSOP engagement
- Long-term medication adherence
Documented informed consent ensures patients understand both benefits and risks, supporting shared decision-making and treatment adherence.
Monitoring, Continuation Criteria, and Treatment Outcomes
Once tirzepatide treatment begins, structured monitoring protocols determine continuation eligibility and optimize outcomes.
Titration Schedule and Monthly Reviews
Tirzepatide follows a gradual dose escalation protocol to minimize gastrointestinal adverse effects:
Standard Titration Schedule:
- Weeks 1-4: 2.5 mg once weekly
- Weeks 5-8: 5 mg once weekly
- Weeks 9-12: 7.5 mg once weekly
- Weeks 13-16: 10 mg once weekly
- Weeks 17-20: 12.5 mg once weekly
- Week 21+: 15 mg once weekly (maximum maintenance dose)
Monthly Review Requirements During Titration:
- Weight measurement and BMI calculation
- Blood pressure and heart rate monitoring
- Adverse effect assessment and management
- Medication adherence review
- BSOP engagement verification
- Dose adjustment decisions based on tolerability and response
These frequent early contacts enable rapid identification of treatment intolerance, optimization of dosing, and reinforcement of behavioural interventions.
Six-Month Assessment and Continuation Criteria
The 6-month review represents a critical decision point for treatment continuation. NHS England requires:
Minimum Weight Loss Threshold:
- ✅ At least 5% reduction from baseline weight required to continue
- ❌ Patients not achieving 5% weight loss should discontinue tirzepatide
- 📊 Weight loss percentage calculated as: [(baseline weight – current weight) / baseline weight] × 100
Additional Continuation Considerations:
- Improvement in obesity-related comorbidities (HbA1c, blood pressure, lipids)
- Tolerability and adverse effect profile
- Treatment adherence and BSOP engagement
- Patient-reported quality of life improvements
- Absence of safety concerns or contraindications
Patients not meeting continuation criteria may be offered alternative weight management approaches, including other medications, specialist obesity services referral, or intensive lifestyle interventions.
Long-Term Monitoring and Review Intervals
After successful 6-month assessment, ongoing monitoring continues at reduced frequency:
3-Monthly Reviews (Months 9, 12, 15, etc.):
- Weight and BMI tracking
- Blood pressure and cardiovascular risk factor monitoring
- Adverse effect surveillance
- Medication adherence assessment
- BSOP engagement review
Annual Comprehensive Assessment:
- Full physical examination
- Repeat laboratory investigations (HbA1c, lipids, renal function)
- Cardiovascular risk recalculation
- Treatment benefit-risk reassessment
- Discussion of treatment duration and future plans
Treatment Duration Considerations: Current NHS guidance does not specify maximum treatment duration, recognizing obesity as a chronic condition requiring long-term management. However, ongoing treatment requires:
- Sustained clinical benefit (maintained weight loss, improved comorbidities)
- Good tolerability without significant adverse effects
- Continued patient engagement with lifestyle modifications
- Absence of emerging contraindications or safety concerns
Alternative Pathways and Private Prescription Options

While NHS access follows strict tirzepatide prescription UK criteria, alternative pathways exist for patients not meeting eligibility requirements or unable to access NHS provision.
Private Prescription Considerations
Private healthcare providers may prescribe tirzepatide with different eligibility criteria, typically including:
Broader BMI Thresholds:
- Often available for BMI ≥30 kg/m² with one obesity-related condition
- May be prescribed for BMI ≥27 kg/m² with significant comorbidities
- Individual provider discretion based on clinical assessment
Fewer Comorbidity Requirements:
- May not require 4 of 5 qualifying conditions
- Clinical judgment based on overall health risk profile
- Consideration of previous weight loss attempt failures
Costs and Accessibility:
- Monthly medication costs typically £150-250 depending on dose
- Additional consultation and monitoring fees
- No BSOP requirement, though behavioural support recommended
- Faster access without NHS waiting lists
Important Considerations:
- Same contraindications apply (pregnancy, MTC history, MEN2)
- Requires ongoing monitoring and follow-up
- Patient self-funds all treatment and monitoring costs
- Quality and safety standards should match NHS provision
Research and Clinical Trial Opportunities
Some patients may access tirzepatide through:
Clinical Research Studies:
- Ongoing trials investigating new indications or formulations
- Usually provide medication at no cost with comprehensive monitoring
- Strict inclusion/exclusion criteria specific to study protocols
- Contribution to advancing medical knowledge
Expanded Access Programmes:
- Occasionally available for specific patient populations
- Typically for patients with exceptional clinical need
- Requires specialist referral and manufacturer approval
For research institutions conducting peptide-related investigations requiring high-purity reference compounds, PEPTIDE PRO’s extensive catalogue includes research-grade materials with full analytical documentation.
Safety Considerations and Adverse Effect Management
Understanding the safety profile of tirzepatide enables informed decision-making and appropriate adverse effect management.
Common Adverse Effects and Management Strategies
Gastrointestinal Symptoms (Most Common):
- Nausea: Affects 20-30% of patients, usually mild-moderate, improves over time
- Management: Eat smaller, more frequent meals; avoid fatty/spicy foods; consider anti-emetics
- Diarrhoea: Occurs in 15-20% of patients
- Management: Maintain hydration; avoid trigger foods; consider loperamide if severe
- Constipation: Affects 10-15% of patients
- Management: Increase dietary fibre; maintain hydration; gentle laxatives if needed
- Vomiting: Less common but may occur during titration
- Management: Temporary dose reduction; anti-emetic medication; ensure adequate hydration
Injection Site Reactions:
- Mild redness, swelling, or itching at injection sites
- Usually resolve within days
- Rotate injection sites to minimize reactions
Metabolic Effects:
- Hypoglycaemia: Risk increased when combined with insulin or sulfonylureas
- Management: Reduce doses of other diabetes medications; patient education about symptoms
- Decreased appetite: Expected effect but may be excessive
- Management: Ensure adequate nutrition; monitor for nutrient deficiencies
Serious Adverse Effects Requiring Immediate Attention
Pancreatitis (Rare but Serious):
- ⚠️ Severe persistent abdominal pain radiating to back
- ⚠️ Nausea and vomiting
- Action: Discontinue tirzepatide immediately; seek urgent medical assessment
Gallbladder Disease:
- ⚠️ Right upper quadrant abdominal pain
- ⚠️ Jaundice (yellowing of skin/eyes)
- Action: Medical evaluation; imaging if indicated; may require treatment discontinuation
Severe Allergic Reactions (Very Rare):
- ⚠️ Difficulty breathing, swelling of face/throat
- ⚠️ Severe rash or hives
- Action: Emergency medical attention; discontinue medication
Thyroid Concerns:
- ⚠️ Lump or swelling in neck
- ⚠️ Hoarseness, difficulty swallowing
- Action: Medical evaluation; thyroid ultrasound if indicated
Acute Kidney Injury:
- ⚠️ Usually secondary to severe dehydration from vomiting/diarrhoea
- Prevention: Maintain hydration during gastrointestinal symptoms; monitor renal function
Drug Interactions and Medication Adjustments
Diabetes Medications:
- Insulin and sulfonylureas require dose reduction to prevent hypoglycaemia
- SGLT2 inhibitors and metformin typically continued without adjustment
- Close glucose monitoring during tirzepatide initiation
Oral Medications:
- Tirzepatide delays gastric emptying, potentially affecting absorption
- Contraceptive pills, thyroid medications, and other critical drugs may require timing adjustments
- Discuss all medications with prescriber
Warfarin:
- May require INR monitoring adjustments due to dietary changes and weight loss
- Regular monitoring recommended
Frequently Asked Questions About Tirzepatide Prescription UK Criteria
Can I get tirzepatide if I only have 3 qualifying conditions?
Currently, only patients in Cohort 3 (starting April 2027) can access tirzepatide with 3 qualifying conditions, and they must have BMI ≥40 kg/m² (or ≥37.5 kg/m² for eligible ethnic groups). Cohorts 1 and 2 require at least 4 of 5 qualifying conditions. Private prescription may offer more flexible criteria.
What happens if I lose 4% weight at 6 months instead of 5%?
The NHS continuation criterion is strict: minimum 5% weight loss required. Patients achieving 4% would typically have treatment discontinued, though individual clinical circumstances may be considered. Discuss your specific situation with your prescriber, as factors like significant comorbidity improvements might influence decisions.
Does my GP practice participate in the Obesity Local Enhanced Service?
Contact your GP practice directly to confirm participation. Not all practices have signed up to the service. If your practice doesn’t participate, you may need to:
- Request they join the service (if feasible)
- Register with a participating practice
- Seek private prescription
- Access specialist obesity services through referral
How long will I need to take tirzepatide?
Obesity is a chronic condition, and current evidence suggests long-term or indefinite treatment may be necessary to maintain weight loss. Many patients regain weight after discontinuation. Treatment duration decisions should be individualized based on response, tolerability, and ongoing benefit-risk assessment.
Can I access tirzepatide if I’m pregnant or planning pregnancy?
No. Tirzepatide is contraindicated in pregnancy and should be discontinued 2 months before planned conception. Females of childbearing potential must use effective contraception during treatment. If pregnancy occurs, stop tirzepatide immediately and inform your healthcare provider.
What if I can’t tolerate the gastrointestinal side effects?
Management strategies include:
- Slower dose titration (staying at lower doses longer)
- Dietary modifications (smaller meals, avoiding triggers)
- Anti-emetic or anti-diarrhoeal medications
- Temporary dose reduction
If symptoms remain intolerable despite these measures, treatment discontinuation may be necessary. Discuss persistent side effects with your prescriber promptly.
Will I need to continue BSOP forever?
While intensive BSOP engagement is mandatory during initial treatment, long-term support requirements vary by local service. Many programmes transition to maintenance support with reduced frequency. Continued lifestyle modification remains essential for sustained weight loss, whether through formal programmes or self-directed approaches.
Can I switch from semaglutide to tirzepatide on the NHS?
Switching between GLP-1 medications requires clinical justification and depends on:
- Your current treatment response
- Eligibility for tirzepatide under NHS criteria
- Local prescribing policies
- Available capacity within the phased rollout
Discuss switching options with your prescriber if you’re currently on semaglutide but believe tirzepatide might offer additional benefit.
Conclusion: Navigating Tirzepatide Access in 2026
Understanding the tirzepatide prescription UK criteria is essential for patients and healthcare professionals navigating this complex but potentially transformative treatment pathway. The strict eligibility requirements—BMI thresholds adjusted for ethnicity, multiple qualifying comorbidities, mandatory behavioural support, and comprehensive monitoring—reflect NHS England’s commitment to delivering maximum clinical benefit while managing substantial resource implications.
The phased rollout continues through 2027, gradually expanding access from the highest-risk patients (Cohort 1) to broader populations (Cohorts 2 and 3). While the 12-year timeline for universal access presents challenges, the structured approach enables sustainable implementation with appropriate safety monitoring and infrastructure development.
Key Action Steps for Patients
If you believe you may be eligible for tirzepatide:
- Verify your GP practice participates in the Obesity Local Enhanced Service
- Calculate your BMI and identify which qualifying conditions you have
- Determine your cohort eligibility based on current phase timelines
- Schedule a comprehensive assessment with your GP to discuss eligibility
- Prepare for BSOP engagement as a mandatory treatment component
- Consider private options if NHS access isn’t available or timely
Key Action Steps for Healthcare Professionals
For prescribers and practice teams:
- Ensure practice registration with the Obesity Local Enhanced Service
- Establish standardized assessment protocols following NHS guidance
- Develop BSOP partnerships or internal programmes
- Implement monitoring systems for baseline, continuation, and long-term reviews
- Educate patients about realistic expectations, adverse effects, and lifestyle requirements
- Maintain comprehensive documentation of eligibility, counselling, and monitoring
The evidence supporting tirzepatide’s efficacy is compelling, with average weight loss of 15-20% representing a significant advancement in obesity pharmacotherapy. However, medication alone cannot address the complex, multifactorial nature of obesity. The NHS pathway’s integration of behavioural support, comprehensive monitoring, and continuation criteria based on demonstrated response reflects evidence-based best practice.
As the rollout continues and real-world experience accumulates, the tirzepatide prescription UK criteria may evolve. Staying informed about updates to eligibility requirements, cohort timelines, and local service availability ensures patients and professionals can make informed decisions about this important treatment option.
For research institutions investigating peptide therapeutics and requiring high-purity reference compounds for scientific studies, PEPTIDE PRO provides research-grade materials with comprehensive quality documentation and rapid UK delivery. All products are clearly labelled for research use only and comply with appropriate regulatory frameworks.
Whether accessing tirzepatide through NHS pathways or exploring alternative options, the comprehensive assessment, monitoring, and lifestyle modification components remain essential for safe, effective treatment and sustained weight management outcomes.
