The pharmaceutical landscape is witnessing a remarkable evolution in metabolic disease treatment, and retatrutide stands at the forefront of this revolution. As researchers and laboratories worldwide monitor the retatrutide FDA approval timeline, understanding the regulatory pathway, clinical milestones, and projected approval dates has become essential for planning future research protocols. This triple agonist peptide has demonstrated unprecedented efficacy in clinical trials, prompting intense scrutiny of its progression through the FDA approval process and its potential availability for research applications.
Key Takeaways
- Retatrutide’s FDA approval timeline is progressing through Phase 3 clinical trials in 2026, with potential NDA submission anticipated in late 2026 or early 2027
- The triple agonist mechanism (GIP/GLP-1/glucagon receptor) distinguishes retatrutide from existing therapies, contributing to superior weight loss and metabolic outcomes in clinical studies
- Research-grade retatrutide is currently available from specialized suppliers like PEPTIDE PRO for laboratory and research purposes only
- FDA approval projections suggest 2027-2028 as the realistic timeframe for commercial authorization, pending successful Phase 3 completion and regulatory review
- Understanding the approval timeline helps researchers plan studies, allocate resources, and anticipate when approved formulations may become available for expanded research protocols
Understanding Retatrutide: The Triple Agonist Breakthrough
Retatrutide represents a significant advancement in peptide therapeutics, functioning as a triple receptor agonist that simultaneously activates glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), and glucagon receptors. This unique mechanism of action differentiates it from single or dual agonists currently available in the metabolic disease treatment landscape.
Mechanism of Action and Research Significance
The triple agonist approach offers several theoretical advantages that have captured the attention of the research community:
GIP Receptor Activation 🔬
- Enhances insulin secretion in a glucose-dependent manner
- Modulates fat metabolism and energy storage
- Contributes to improved glycemic control
GLP-1 Receptor Activation 💊
- Reduces appetite and food intake
- Slows gastric emptying
- Improves insulin sensitivity and beta-cell function
Glucagon Receptor Activation ⚡
- Increases energy expenditure
- Promotes fat oxidation
- Enhances metabolic rate
This comprehensive receptor engagement explains the exceptional results observed in clinical trials, where retatrutide has demonstrated weight loss outcomes exceeding those of existing GLP-1 receptor agonists. For researchers interested in studying multi-receptor peptide mechanisms, research-grade peptides provide essential tools for laboratory investigation.
Clinical Trial Results Driving FDA Interest
The robust clinical data supporting retatrutide has accelerated regulatory interest and shaped the FDA approval timeline. Key findings include:
| Trial Phase | Weight Loss Results | Metabolic Improvements | Safety Profile |
|---|---|---|---|
| Phase 1 | Dose-dependent efficacy | Improved glucose control | Generally well-tolerated |
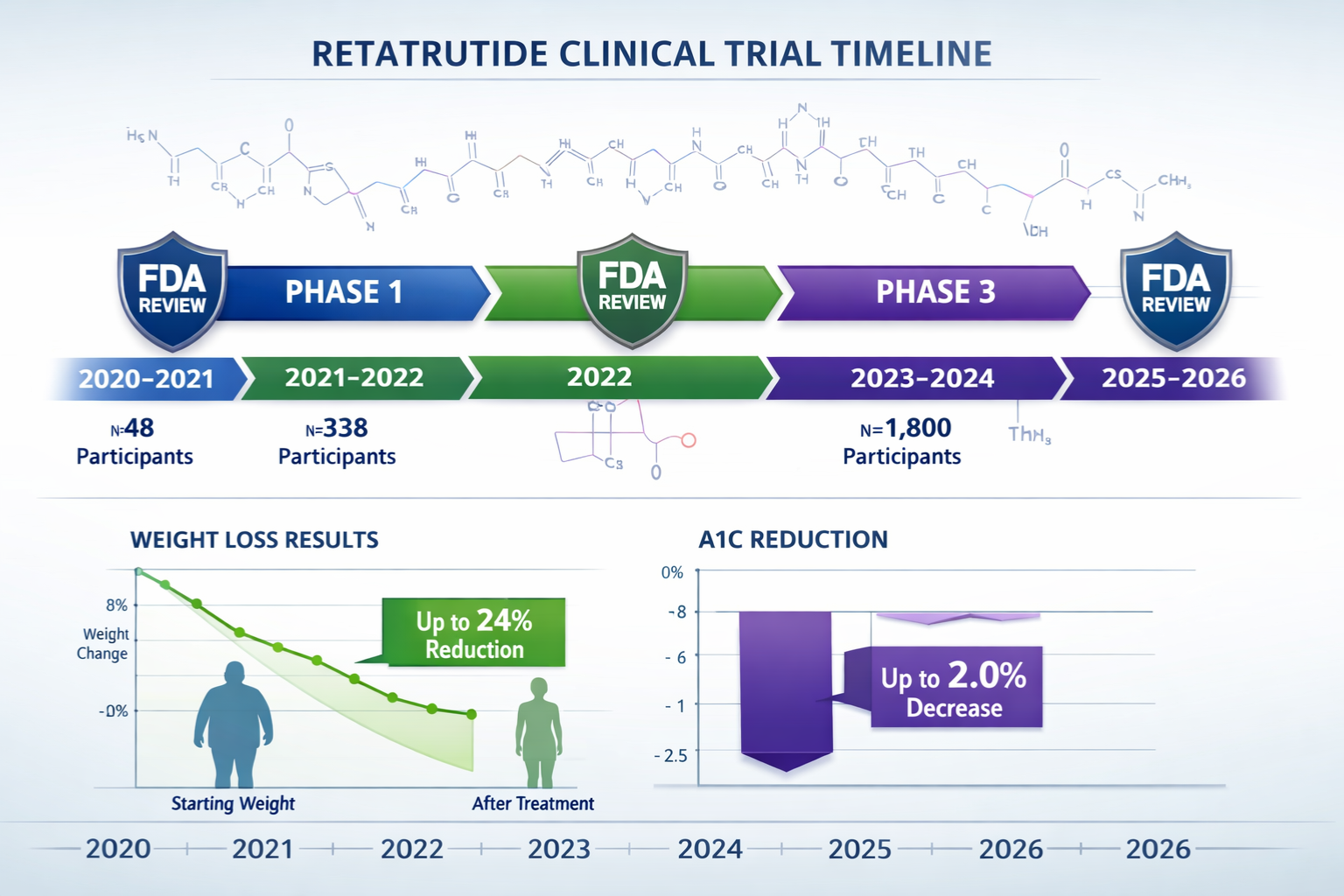
| Phase 2 | Up to 24% body weight reduction | Significant HbA1c reduction | Manageable GI side effects |
| Phase 3 (ongoing) | Expected similar or superior | Comprehensive metabolic benefits | Long-term safety monitoring |
These impressive outcomes have positioned retatrutide as a potential best-in-class therapy, intensifying focus on its regulatory progression and eventual approval.
The Retatrutide FDA Approval Timeline: Current Status in 2026
Understanding where retatrutide stands in the FDA approval process requires examining the standard regulatory pathway and the specific milestones achieved to date. The retatrutide FDA approval timeline follows a well-established sequence that ensures safety, efficacy, and manufacturing quality before any therapeutic agent reaches the market.
Standard FDA Approval Pathway for Novel Peptides
The FDA approval process for innovative peptide therapeutics like retatrutide typically encompasses several critical stages:
1. Preclinical Development 🧪
- Laboratory research and animal studies
- Mechanism of action characterization
- Initial safety and toxicology assessment
- Duration: 3-6 years (completed for retatrutide)
2. Investigational New Drug (IND) Application 📋
- Submission of preclinical data to FDA
- Proposed clinical trial protocols
- Manufacturing and quality control information
- FDA review period: 30 days (completed)
3. Phase 1 Clinical Trials 👥
- First-in-human studies (20-100 participants)
- Safety, tolerability, and pharmacokinetics
- Dose-ranging studies
- Duration: 1-2 years (completed for retatrutide)
4. Phase 2 Clinical Trials 📊
- Efficacy and safety in target population (100-500 participants)
- Optimal dosing determination
- Preliminary efficacy signals
- Duration: 2-3 years (completed for retatrutide)
5. Phase 3 Clinical Trials 🏥
- Large-scale efficacy and safety studies (1,000-5,000+ participants)
- Confirmatory evidence for approval
- Long-term safety monitoring
- Duration: 2-4 years (currently ongoing for retatrutide in 2026)
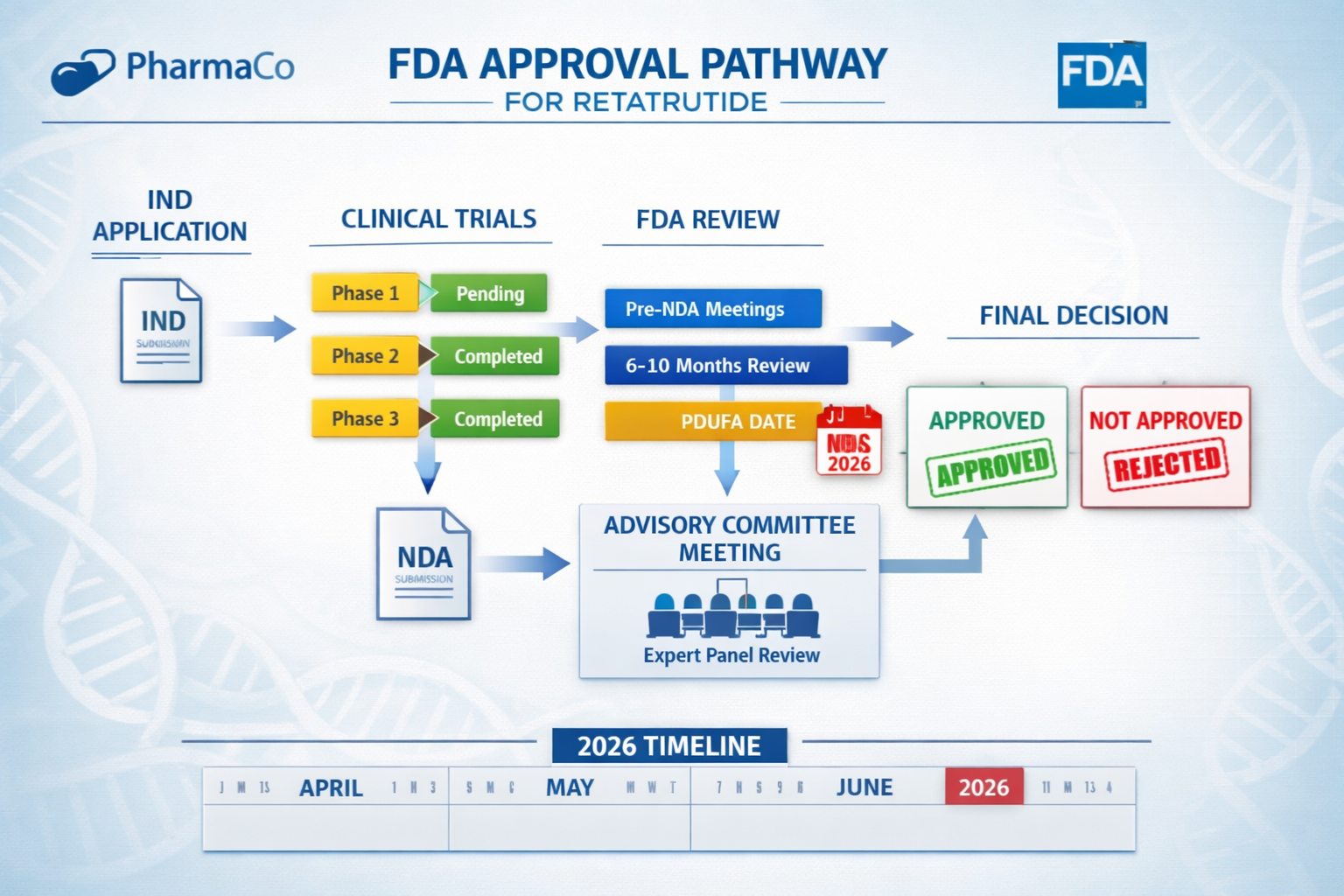
6. New Drug Application (NDA) Submission 📄
- Comprehensive data package to FDA
- All clinical, preclinical, and manufacturing data
- Proposed labeling and risk management
- Anticipated for retatrutide: Late 2026 or 2027
7. FDA Review Process 🔍
- Standard review: 10 months
- Priority review: 6 months (if granted)
- Advisory committee meetings (if required)
- FDA decision and potential approval
Retatrutide’s Current Position: 2026 Update
As of 2026, retatrutide has successfully completed Phase 1 and Phase 2 clinical trials with remarkable results. The developer, Eli Lilly and Company, initiated multiple Phase 3 trials examining retatrutide’s efficacy in:
- Obesity management (participants with BMI ≥30 or ≥27 with comorbidities)
- Type 2 diabetes treatment (glycemic control and weight management)
- Metabolic dysfunction-associated steatohepatitis (MASH)
- Cardiovascular outcomes (long-term safety and benefits)
These comprehensive Phase 3 programs represent the final major hurdle before NDA submission. The trials are designed to enroll thousands of participants across multiple countries, providing robust evidence of efficacy and safety in diverse populations.
“The Phase 3 program for retatrutide represents one of the most comprehensive development efforts for a metabolic disease therapy, reflecting both the compound’s promise and the rigorous standards required for FDA approval.” — Pharmaceutical industry analyst, 2026
Projected Timeline for FDA Approval
Based on current progress and typical regulatory timelines, the retatrutide FDA approval timeline can be projected as follows:
2026 (Current Year) 📅
- Ongoing Phase 3 clinical trials
- Interim data analysis and safety monitoring
- Preparation of regulatory submission materials
- Engagement with FDA on submission requirements
Late 2026 – Early 2027 📅
- Completion of Phase 3 trials (anticipated)
- Final data analysis and compilation
- NDA submission to FDA (projected)
2027 📅
- FDA acceptance and filing of NDA
- FDA review process (6-10 months)
- Potential Advisory Committee meeting
- Possible FDA approval decision (optimistic scenario)
2027-2028 📅
- More conservative approval timeline
- Additional data requests or clarifications
- Final FDA decision and approval (realistic scenario)
Post-Approval 📅
- Phase 4 post-marketing surveillance
- Real-world evidence collection
- Potential label expansions for additional indications
It’s important to note that these projections are subject to change based on trial outcomes, regulatory feedback, and unforeseen developments. Researchers planning studies involving retatrutide should maintain flexibility in their timelines and consider working with research-grade formulations currently available for laboratory investigation.
Factors That Could Accelerate or Delay Approval
Several variables may influence the final retatrutide FDA approval timeline:
Potential Accelerators ⚡
- Priority Review designation: If FDA grants priority review status based on significant therapeutic advancement
- Breakthrough Therapy designation: Already granted for certain indications, providing enhanced FDA guidance
- Exceptional efficacy data: Superior results compared to existing therapies
- Unmet medical need: Growing obesity and metabolic disease epidemic
- Clean safety profile: Absence of unexpected adverse events
Potential Delays ⏸️
- Safety concerns: Emergence of unexpected adverse events in Phase 3
- Manufacturing issues: Quality control or supply chain challenges
- Incomplete data: Need for extended follow-up periods
- Regulatory questions: FDA requests for additional studies or analyses
- Advisory Committee concerns: Unexpected issues raised during review
Researchers monitoring the approval process should stay informed through official FDA communications, clinical trial registries, and pharmaceutical company announcements to adjust their research plans accordingly.
Implications for Research: Accessing Retatrutide Before FDA Approval
While the commercial approval of retatrutide remains on the horizon, the research community requires access to high-quality peptide compounds for ongoing scientific investigation. Understanding the distinction between FDA-approved therapeutics and research-grade materials is essential for laboratories conducting studies in metabolic disease, receptor pharmacology, and peptide therapeutics.
Research-Grade Retatrutide: Current Availability
Research-grade retatrutide is currently available through specialized peptide suppliers who provide compounds explicitly labeled “For Research Use Only” (RUO). These materials serve critical functions in:
🔬 Mechanistic Studies
- Investigating triple receptor agonist mechanisms
- Comparing efficacy with single or dual agonists
- Characterizing receptor binding and activation profiles
🧬 Preclinical Research
- Animal model studies of obesity and metabolic disease
- Pharmacokinetic and pharmacodynamic characterization
- Toxicology and safety assessments
📊 Analytical Method Development
- Developing assays for peptide quantification
- Stability testing under various conditions
- Quality control methodology establishment
�
� Formulation Research
- Investigating optimal delivery systems
- Studying peptide stability and degradation
- Developing novel administration routes
PEPTIDE PRO offers research-grade retatrutide formulations with comprehensive quality documentation, including Certificates of Analysis (COA) that verify purity and identity. These research materials enable laboratories to conduct studies without waiting for final FDA approval of commercial formulations.
Quality Considerations for Research Peptides
When selecting research-grade retatrutide, several quality parameters should be evaluated:
Purity Standards ✅
- High-performance liquid chromatography (HPLC) purity ≥98%
- Mass spectrometry confirmation of molecular weight
- Absence of significant impurities or degradation products
Storage and Handling ❄️
- Lyophilized (freeze-dried) format for stability
- Storage at -20°C or lower
- Protection from light and moisture
- Clear reconstitution guidelines
Documentation 📋
- Certificate of Analysis (COA) with each batch
- Detailed product specifications
- Storage and handling recommendations
- Regulatory compliance statements (RUO designation)
Supplier Reliability 🏆
- Established reputation in research community
- Consistent product quality across batches
- Responsive customer support
- Fast, reliable delivery with appropriate packaging
PEPTIDE PRO maintains strict quality control standards for all research peptides, ensuring that materials meet the exacting requirements of scientific investigation. Their extensive peptide catalogue includes retatrutide alongside other metabolic research compounds like semaglutide and tirzepatide.
Regulatory Compliance for Research Use
Researchers must understand and comply with regulations governing research-grade materials:
Clear Labeling Requirements
🏷
️
- All research peptides must be clearly labeled “For Research Use Only”
- Not for human or animal consumption
- Not intended for diagnostic or therapeutic purposes
Institutional Oversight
🏛
️
- Research protocols should be reviewed by institutional committees
- Animal studies require IACUC approval
- Proper documentation of research use
Storage and Disposal ♻️
- Secure storage in research facilities
- Proper chain of custody documentation
- Appropriate disposal following institutional protocols
Import/Export Considerations 🌍
- Compliance with customs regulations
- Proper documentation for international shipments
- Understanding country-specific research material regulations
By maintaining strict adherence to these guidelines, researchers can confidently utilize research-grade retatrutide while awaiting FDA approval of commercial formulations.
Comparing Research-Grade and FDA-Approved Formulations
Understanding the differences between research-grade peptides and eventual FDA-approved products helps researchers plan long-term study designs:
| Aspect | Research-Grade Retatrutide | FDA-Approved Retatrutide (Future) |
|---|---|---|
| Intended Use | Laboratory research only | Clinical/therapeutic use |
| Regulatory Status | Not FDA-approved | Full FDA approval |
| Quality Standards | High purity (≥98%) | GMP manufacturing standards |
| Documentation | COA, specifications | Complete prescribing information |
| Availability | Currently available | Post-approval (2027-2028 projected) |
| Cost | Research pricing | Commercial pharmaceutical pricing |
| Formulation | Lyophilized powder | Standardized clinical formulation |
Researchers conducting studies with research-grade retatrutide should recognize that findings may inform but not directly translate to clinical use until FDA approval is granted and commercial formulations become available.
Monitoring the Retatrutide FDA Approval Timeline: Resources for Researchers
Staying informed about the retatrutide FDA approval timeline requires accessing reliable, up-to-date information from authoritative sources. Researchers can utilize several resources to track regulatory progress and plan their studies accordingly.
Official FDA Resources
FDA Drugs Database 🔍
- Search for retatrutide by generic or brand name
- View application status and approval history
- Access approved labeling and safety information
- Monitor FDA communications and updates
FDA News and Events 📰
- Press releases announcing approvals or significant actions
- Advisory Committee meeting schedules and materials
- Public hearing announcements
- Safety communications and alerts
ClinicalTrials.gov 📊
- Comprehensive database of ongoing clinical trials
- Search for retatrutide trials by phase, indication, or sponsor
- View trial design, enrollment status, and preliminary results
- Monitor trial completion dates and data availability
Pharmaceutical Company Communications
Eli Lilly Investor Relations 💼
- Quarterly earnings calls with pipeline updates
- Press releases on clinical trial milestones
- Regulatory submission announcements
- Anticipated approval timelines
Scientific Conferences 🎓
- Presentation of clinical trial data at major conferences
- American Diabetes Association (ADA) meetings
- Obesity Week conferences
- European Association for the Study of Diabetes (EASD)
Scientific Literature and Publications
Peer-Reviewed Journals 📚
- Publication of Phase 2 and Phase 3 trial results
- Mechanism of action studies
- Safety and efficacy analyses
- Comparative effectiveness research
Key Journals to Monitor:
- New England Journal of Medicine
- The Lancet
- JAMA
- Diabetes Care
- Obesity
Industry Analysis and News Sources
Pharmaceutical Industry Publications 📱
- FiercePharma
- BioPharma Dive
- Endpoints News
- STAT News
These sources provide timely reporting on regulatory developments, clinical trial results, and approval timeline updates.
Research Community Networks
Professional Organizations 🤝
- Peptide Society meetings and publications
- Endocrine Society communications
- Obesity Society updates
- American Diabetes Association resources
Research Forums and Discussion Groups 💬
- Scientific social media platforms
- Research-focused LinkedIn groups
- Academic institution newsletters
- Laboratory supplier communications
PEPTIDE PRO maintains communication with the research community through regular updates on peptide availability, regulatory developments, and scientific advances relevant to metabolic research.
Strategic Planning: Preparing for Post-Approval Research Opportunities
As the retatrutide FDA approval timeline progresses toward anticipated approval in 2027-2028, forward-thinking researchers should begin planning for the expanded research opportunities that will emerge once commercial formulations become available.
Research Areas Likely to Expand Post-Approval
Comparative Effectiveness Studies 📊
- Head-to-head comparisons with approved GLP-1 agonists
- Cost-effectiveness analyses
- Quality of life assessments
- Long-term outcomes research
Mechanism of Action Research 🔬
- Detailed receptor pharmacology studies
- Tissue-specific effects characterization
- Metabolic pathway mapping
- Biomarker identification
Combination Therapy Investigations 💊
- Synergistic effects with other metabolic agents
- Optimal combination strategies
- Safety profiles of multi-drug regimens
- Personalized medicine approaches
Special Populations 👥
- Pediatric obesity research (if approved)
- Geriatric population studies
- Diverse ethnic and genetic backgrounds
- Comorbidity-specific investigations
Novel Indications
�
�
- Metabolic dysfunction-associated conditions
- Cardiovascular disease prevention
- Neurodegenerative disease connections
- Cancer metabolism research
Building Research Infrastructure
Laboratories planning to conduct retatrutide research post-approval should consider:
Analytical Capabilities 🧪
- HPLC and mass spectrometry equipment
- Receptor binding assay systems
- Metabolic measurement tools
- Biomarker analysis platforms
Collaborative Networks 🤝
- Partnerships with clinical research centers
- Multi-institutional study consortia
- Industry collaboration opportunities
- Patient advocacy group connections
Funding Strategies 💰
- Grant applications aligned with approval timeline
- Industry-sponsored research opportunities
- Foundation and society funding programs
- Institutional research support
Regulatory Preparation 📋
- IRB protocols for human studies (if applicable)
- IACUC protocols for animal research
- Data management and privacy systems
- Compliance infrastructure
Leveraging Current Research-Grade Materials
While awaiting FDA approval, researchers can maximize productivity by:
Establishing Baseline Data 📈
- Conducting preliminary mechanistic studies
- Developing and validating analytical methods
- Characterizing pharmacological properties
- Building expertise with the compound
Pilot Studies 🧬
- Small-scale investigations to inform larger studies
- Proof-of-concept experiments
- Method optimization and validation
- Preliminary data for grant applications
Comparative Research ⚖️
- Studies comparing retatrutide with other research peptides
- Mechanism differentiation from single or dual agonists
- Structure-activity relationship investigations
- Receptor selectivity characterization
Publication Strategy 📝
- Publishing preclinical findings before approval
- Establishing research expertise and credibility
- Building collaborative networks
- Positioning for post-approval funding opportunities
The Broader Context: Retatrutide in the Evolving Metabolic Therapy Landscape
Understanding the retatrutide FDA approval timeline requires placing it within the broader context of metabolic disease treatment evolution and the competitive landscape of incretin-based therapies.
The Incretin Revolution: From Single to Triple Agonists
The development of incretin-based therapies has progressed through distinct generations:
First Generation: GLP-1 Receptor Agonists 🥇
- Exenatide (Byetta) – 2005 approval
- Liraglutide (Victoza) – 2010 approval
- Semaglutide (Ozempic/Wegovy) – 2017/2021 approvals
- Established efficacy in glycemic control and weight loss
Second Generation: Dual Agonists 🥈
- Tirzepatide (Mounjaro/Zepbound) – 2022/2023 approvals
- GIP/GLP-1 receptor agonist
- Superior weight loss compared to GLP-1-only agonists
- Enhanced metabolic benefits
Third Generation: Triple Agonists 🥉
- Retatrutide (investigational) – anticipated 2027-2028 approval
- GIP/GLP-1/glucagon receptor agonist
- Potential best-in-class efficacy
- Comprehensive metabolic optimization
This progression demonstrates the pharmaceutical industry’s commitment to developing increasingly effective metabolic therapies, with each generation building upon the successes and limitations of its predecessors.
Competitive Landscape and Market Implications
The metabolic therapy market has experienced explosive growth, driven by:
📈 Rising Obesity Prevalence
- Over 40% of adults in developed countries classified as obese
- Growing recognition of obesity as a chronic disease
- Increased healthcare costs associated with metabolic disorders
💰 Market Size and Growth
- GLP-1 agonist market exceeded $20 billion in 2025
- Projected to reach $100+ billion by 2030
- Driving significant pharmaceutical industry investment
🔬 Innovation Pipeline
- Multiple companies developing next-generation metabolic therapies
- Oral formulations in development
- Novel mechanisms beyond incretin pathways
- Personalized medicine approaches emerging
Retatrutide’s position as a potential best-in-class therapy makes its approval timeline particularly significant for both clinical practice and research opportunities.
Implications for Research Peptide Suppliers
The anticipated approval of retatrutide and continued innovation in metabolic peptides creates opportunities for research suppliers like PEPTIDE PRO:
Expanding Research Demand 📊
- Growing interest in metabolic peptide mechanisms
- Increased funding for obesity and diabetes research
- Academic and industry research expansion
- Novel indication exploration
Quality and Reliability Imperative ✅
- Researchers require consistent, high-purity materials
- Documentation and traceability increasingly important
- Fast, reliable delivery essential for time-sensitive studies
- Comprehensive technical support valued by laboratories
Educational Resources 📚
- Researchers need guidance on peptide handling and storage
- Reconstitution protocols and stability information
- Comparative data on different peptide formulations
- Best practices for research applications
PEPTIDE PRO’s commitment to exceptional purity, fast delivery, and professional service positions it as a trusted partner for researchers navigating the evolving metabolic peptide landscape.
Conclusion: Navigating the Retatrutide FDA Approval Timeline
The retatrutide FDA approval timeline represents a critical pathway in the evolution of metabolic disease treatment, with significant implications for both clinical practice and scientific research. As of 2026, retatrutide progresses through Phase 3 clinical trials with anticipated NDA submission in late 2026 or early 2027, positioning it for potential FDA approval in 2027-2028.
Key Points to Remember
✅ Retatrutide’s triple agonist mechanism (GIP/GLP-1/glucagon receptors) offers potential advantages over existing single or dual agonist therapies, with clinical trial data suggesting superior weight loss and metabolic benefits.
✅ The regulatory pathway follows established FDA processes, with current focus on completing comprehensive Phase 3 trials across multiple indications including obesity, type 2 diabetes, and metabolic dysfunction-associated conditions.
✅ Research-grade retatrutide is currently available from specialized suppliers like PEPTIDE PRO, enabling laboratories to conduct mechanistic studies, preclinical research, and method development while awaiting commercial approval.
✅ Projected approval timeline suggests 2027-2028 as the realistic window for FDA authorization, though this remains subject to trial outcomes, regulatory review, and potential unforeseen developments.
✅ Strategic planning for post-approval research opportunities should begin now, including infrastructure development, collaborative network building, and preliminary studies using research-grade materials.
Actionable Next Steps for Researchers
For Laboratories Conducting Metabolic Research:
- Monitor Official Sources 📱
- Track clinical trial progress on ClinicalTrials.gov
- Follow FDA announcements and pharmaceutical company communications
- Subscribe to relevant scientific journals and industry publications
- Access Research-Grade Materials 🔬
- Source high-purity retatrutide from reputable suppliers
- Verify quality documentation (COA, specifications)
- Establish proper storage and handling protocols
- Consider exploring PEPTIDE PRO’s research peptide catalogue
- Build Research Capabilities 🧪
- Develop analytical methods for peptide characterization
- Establish collaborative networks for post-approval studies
- Prepare grant applications aligned with approval timeline
- Conduct preliminary studies to generate pilot data
- Stay Informed and Adaptable 📚
- Attend scientific conferences featuring metabolic peptide research
- Participate in professional organization activities
- Maintain flexibility in research timelines
- Prepare for potential approval acceleration or delays
- Ensure Regulatory Compliance ✅
- Clearly designate all materials as “For Research Use Only”
- Obtain appropriate institutional approvals for studies
- Document research use and maintain proper records
- Follow disposal and safety protocols
The Future of Metabolic Peptide Research
The anticipated approval of retatrutide marks not an endpoint but a new beginning in metabolic disease research. The compound’s unique triple agonist mechanism opens numerous avenues for scientific investigation, from detailed receptor pharmacology to novel therapeutic applications beyond obesity and diabetes.
Researchers who establish expertise with retatrutide during the pre-approval period position themselves advantageously for the expanded opportunities that will emerge post-approval. By working with high-quality research-grade materials, developing robust analytical methods, and building collaborative networks, laboratories can contribute meaningfully to the growing body of knowledge surrounding this promising therapeutic class.
Partner with PEPTIDE PRO for Your Research Needs
As the retatrutide FDA approval timeline progresses toward anticipated authorization, PEPTIDE PRO remains committed to supporting the research community with:
🔬 Premium Research-Grade Peptides – Including retatrutide and comprehensive metabolic research compounds, all produced and handled under strict quality conditions
⚡ Fast, Reliable Delivery – Same-day dispatch for orders placed before 1pm (Mon-Fri), with fast UK delivery and international shipping options
📋 Complete Documentation – Certificates of Analysis, product specifications, and storage guidance with every order
🤝 Professional Support – Expert customer service to answer questions, provide technical guidance, and support your research objectives
💯 Uncompromising Quality – Exceptional purity standards, consistent batch-to-batch reliability, and transparent quality control
Visit PEPTIDE PRO to explore the complete range of research peptides, access educational resources on peptide handling and storage, and connect with a supplier trusted by researchers and laboratories across the UK and worldwide.
Strictly for Research Use Only ⚠️
All peptides discussed in this article, including retatrutide, are available exclusively for research purposes and are clearly labeled “For Research Use Only.” These materials are not for human or animal consumption and are not intended for diagnostic or therapeutic purposes. Researchers must comply with all applicable regulations and institutional guidelines when conducting studies with research-grade peptides.
